Impressive Chemical Equation For Combustion Of Propane

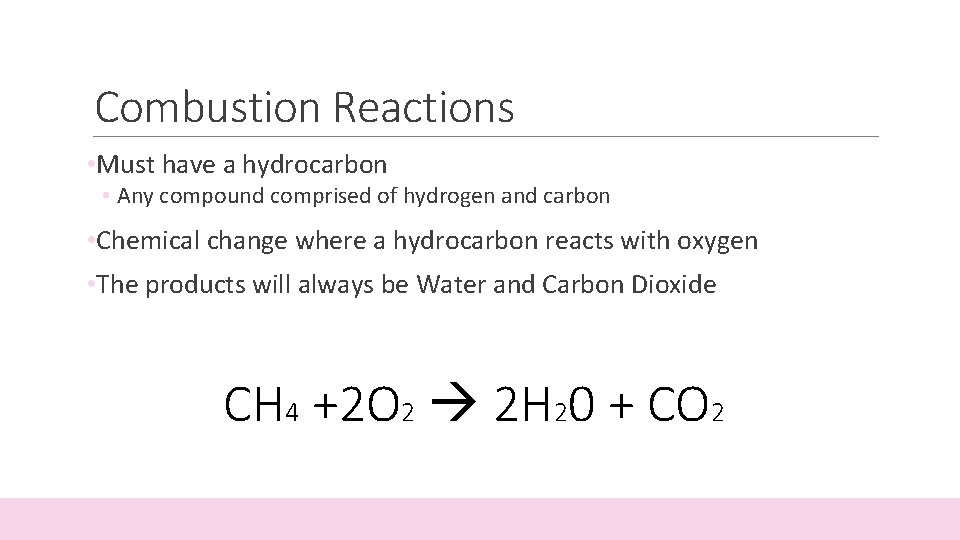
For hydrocarbons that end in the suffix -ane the formula is CnH 2n2.
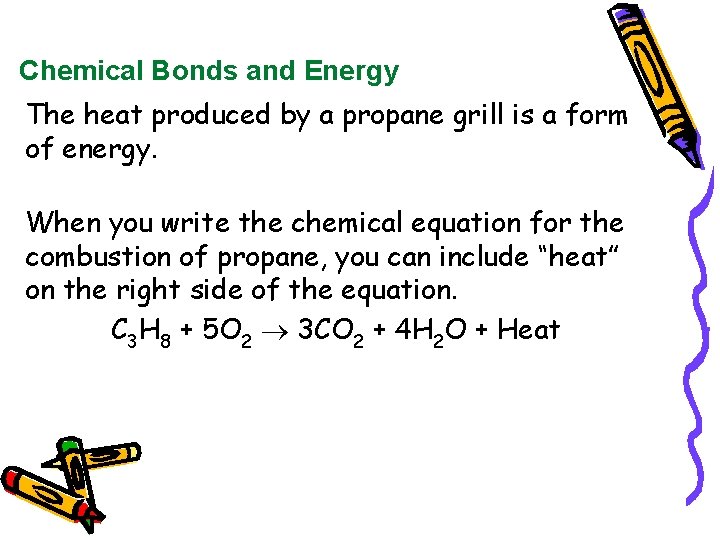
Chemical equation for combustion of propane. Here are the data files csv for different conditions. 2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat. Complete combustion does NOT give carbon monoxide or sootCheck me out.
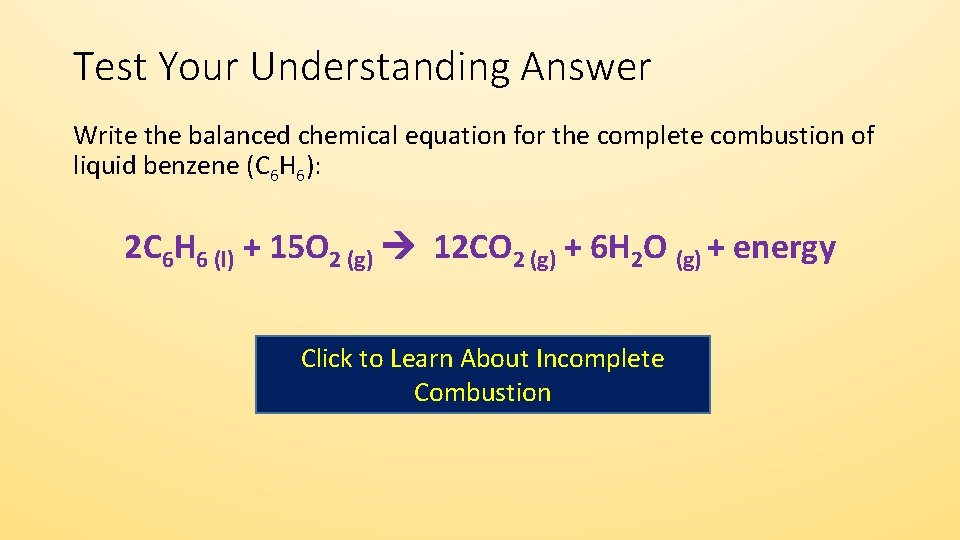
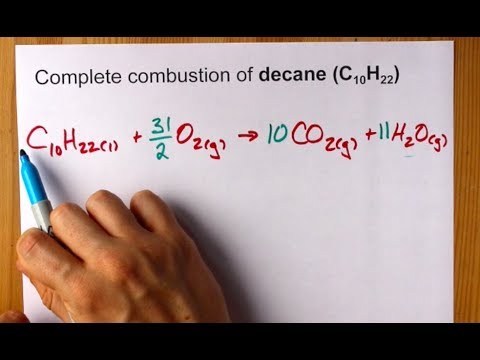
Propane is one of the hydrocarbon components of raw natural gas which is a type of fossil fuel. C3H8 5O2 3CO2 4H2O. 300K - 1bar - phi1.
Write a balanced chemical equation for the combustion of gaseous propane in gaseous oxygen to produce gaseous carbon dioxide and liquid water. 1- Firstly the carbon in the hydrocarbon propane oxidizes with oxygen to produce carbon dioxide. Balancing chemical reaction balancing combustion of propanec3h8o2--co2h2o.
Propane C3H8 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. More can be found on this file. A 1200g H2O are produced.
If not enough oxygen is present for complete combustion incomplete combustion occurs. Incomplete combustion of propane yields carbon carbon dioxide and water. Hydrocarbon oxygen carbon dioxide water Here are the equations for the complete combustion of propane used in bottled gas.
Explained Process of Combustion of Propane. When writing equations with incomplete combustion it is advisable to include only one carbon product otherwise there will be multiple solutions to the equation. Multiple carbon products produce balanced equations with multiple solutions Example.