Fine Beautiful Equation Balancer With Charges

Fe Au Co Br C O N F.
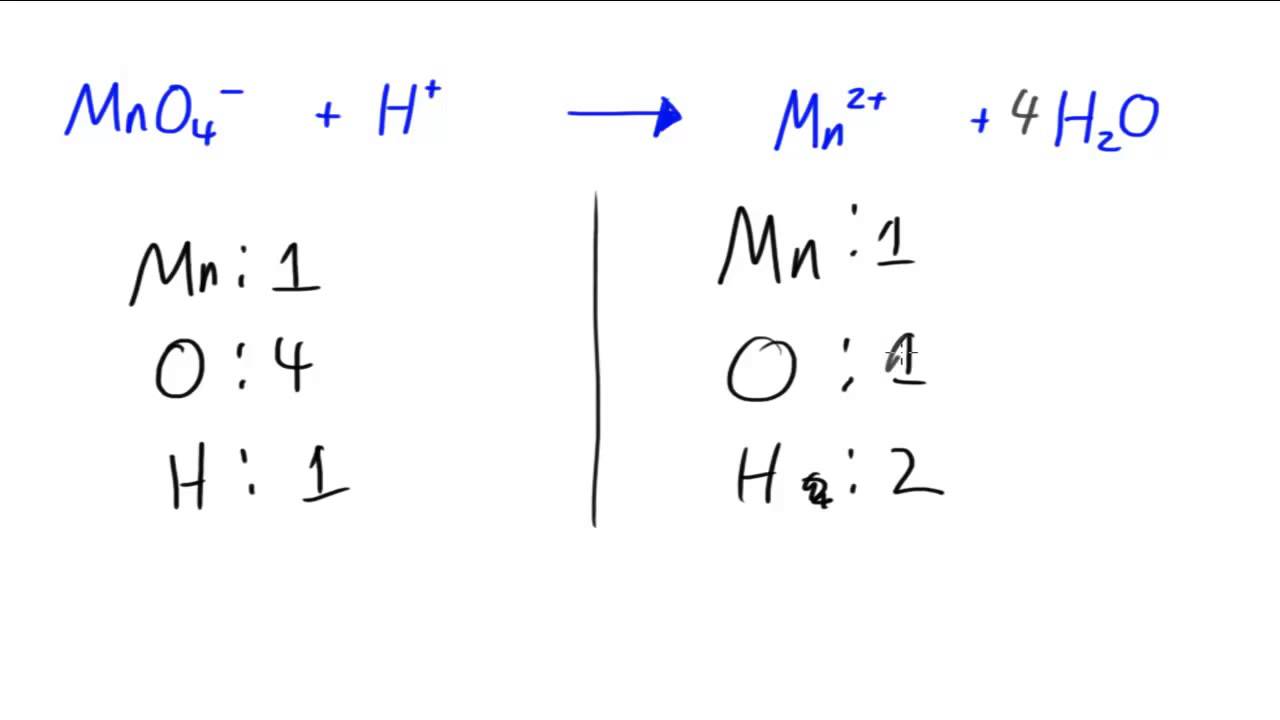
Equation balancer with charges. H 2 O 2 H 2 O Na 2 Cl 2 NaCl. The LHS consists of the reactants and the RHS consists of the products. The net charge must be the same on each side of the equation once it has been balanced.
Change the coefficients the numbers in front of the compound or molecule so that the number of atoms of the element is the same on each side of the equation. Count the equations and unknowns---need same number of eqns as unknowns to solve 6. Do charges matter when balancing equations.
What is the net charge on each side of the equation. The concentration of either protons or hydroxide ions can. This continues to happen until the charges balance and there is no more attraction.
Instructions on balancing chemical equations. Enter a chemical equation to balance Chemical Equations Examples. Write mass balance equations there may be more than one 4.
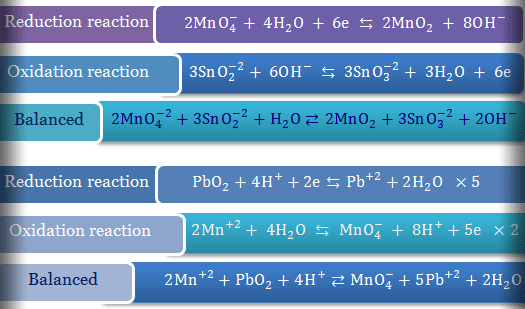
We can only write one complete charge balance for a solution. The charge balance must account for all positively charged sodium and hydronium ions and negatively charged acetate and hydroxide ions species in solution. It is an online tool which works digitally and provide quick results.
Just look at all the ions in our total ionic equation. The first half-reaction has 7 on the left and 2 on the right. This is because positive and negative charges attract each other.