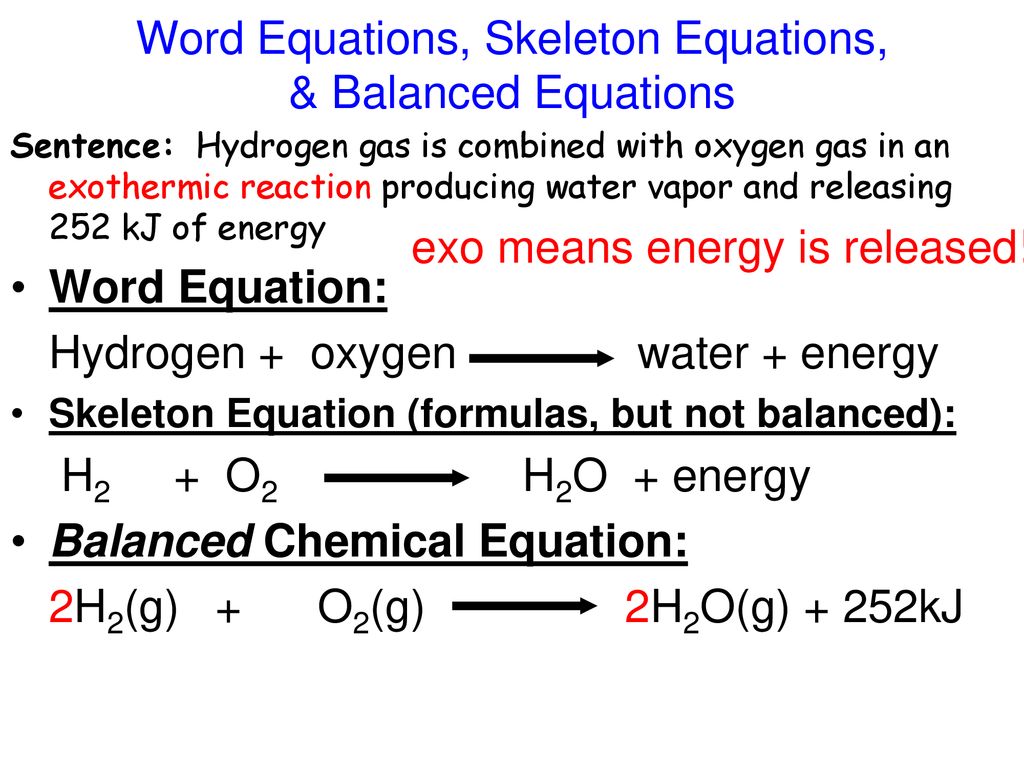
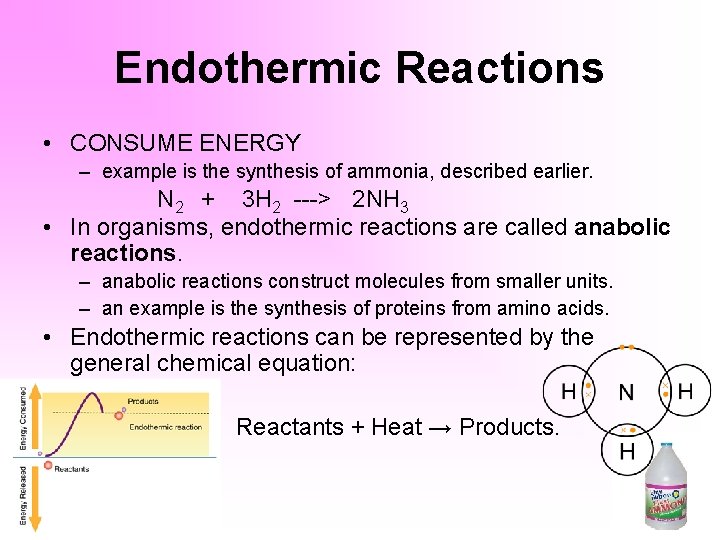
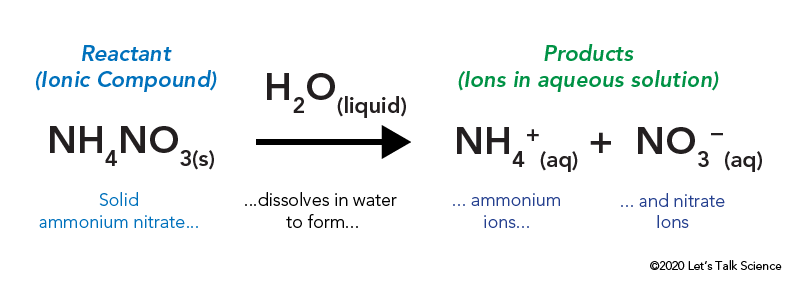
Exemplary Exothermic Reaction Word Equation

The characteristics of an exothermic reaction can be expressed with the general chemical equation.
Exothermic reaction word equation. -Through an ____ in temperature from the reactants to the products. Reactants Products Energy. Q8 Give examples of exothermic reactions ie.
The general equation for an exothermic reaction is. The reaction CH4 H 2 O CO 3H 2 is endothermic ΔH 206 kJmol while CH 4 05 O 2 CO 2H 2 is exothermic ΔH 38 kJmol. Reaction between a solid and a liquid.
Exothermic Nuclear Reaction Definition An exothermic reaction is a chemical reaction that releases energy through light or heat. So an exothermic reaction results in the chemical product and a release of energy. Energy is the ability to do work or to produce heat.
This is illustrated in the Figure below. Exothermic Reaction An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. Give two examples of exothermic reactions seen in everyday life.
In these cases the contact surface is very much increased. - ____ How do you know that an exothermic reaction has taken place. What is the name given to reactions that transfer energy from the surroundings to the reactants.
In a chemical equation the location of the word heat can be used to quickly determine whether the reaction is endothermic or exothermic. Using your equation a negative delta H would indicate an endothermic reaction and a positive delta H would indicate an exothermic reaction. A chemical reaction is a chemical change in which one or more substances react to form new substances with entirely different properties.