Stunning Hydrocarbon Incomplete Combustion Equation
The incomplete combustion of a hydrocarbon usually produces a sooty flame due to the presence of carbon C or soot as a product of the incomplete combustion reaction.
Hydrocarbon incomplete combustion equation. As a simple way of thinking about it the hydrogen in the hydrocarbon gets the first chance at the oxygen and the carbon gets whatever is. When this equation is balanced you get. Tap again to see term.
Or incomplete depending on the availability of the oxygen reactant. The formula for ethanol is given by C 2 H 5 OH. Discover free flashcards games and test prep activities designed to help you learn about Complete Combustion Equation and other concepts.
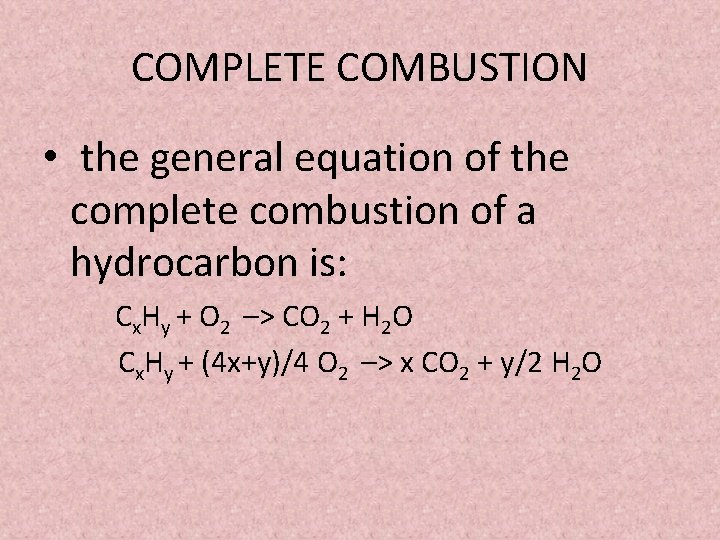
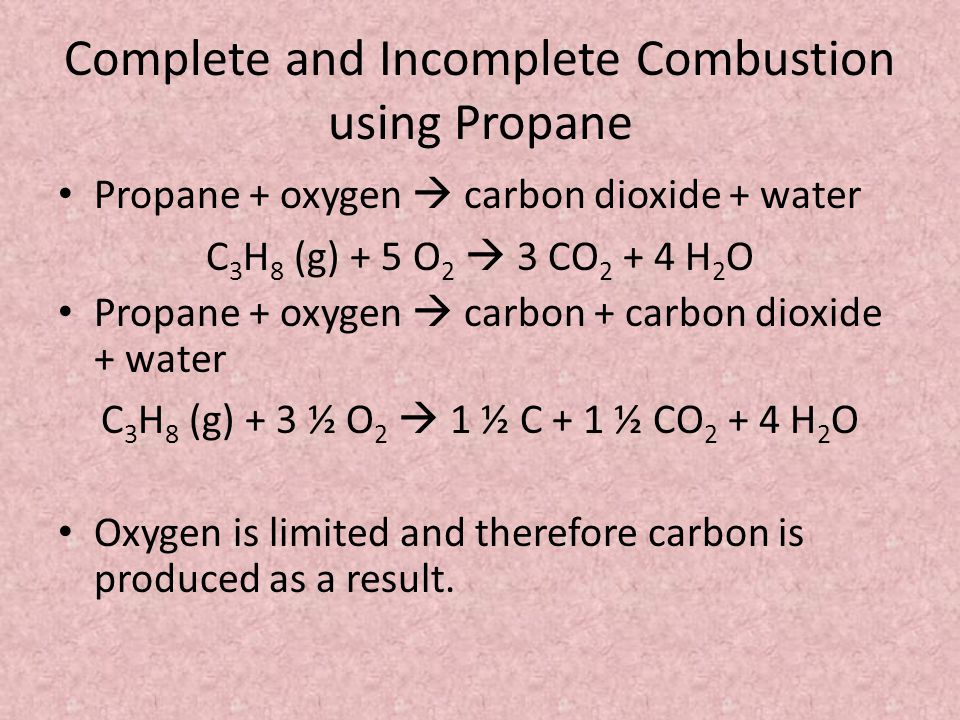
The complete combustion of hydrocarbons leads to carbon dioxide and water formation while incomplete combustion yields carbon dioxide carbon monoxide soot and water. 2-methylbutane is a hydrocarbon molecule. CH 4 2O 2 CO 2 2H 2 O.
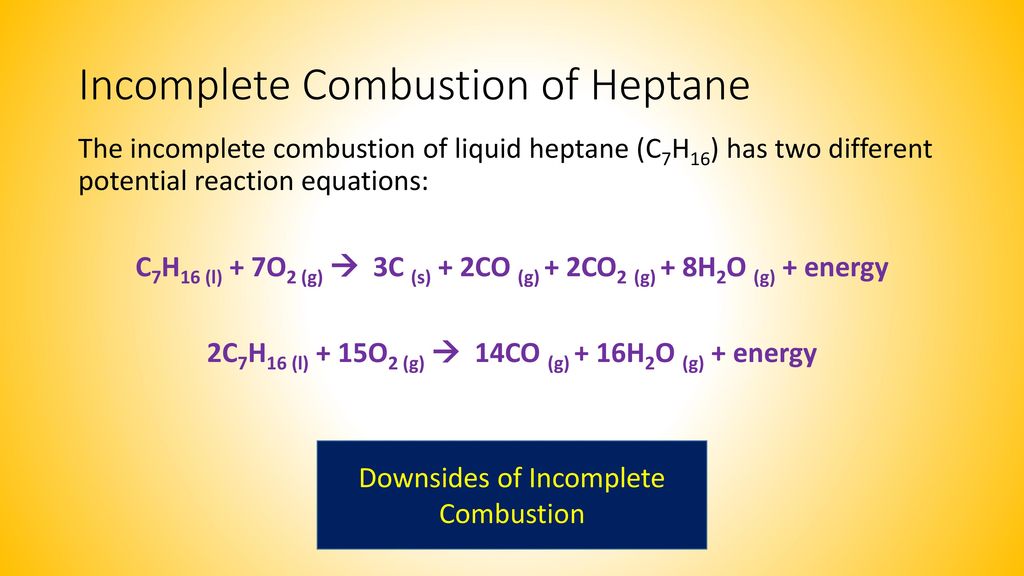
Ethanol is a fuel source in an alcohol lamp. Because so many reaction products are possible incomplete combustion cannot be represented by a single chemical equation. C7H16l 7 O2g 3 Cs 2 COg 2 CO2g 8 H2Og energy.
4CH4 5O2 2CO 8H2O 2C. Incomplete Combustion of Hydrocarbons Chemistry Start Practising In this worksheet we will practice describing the incomplete combustion of hydrocarbons by writing balancing and interpreting chemical equations. Write and balance the equation for the complete combustion of 2-methylbutane.
Methane oxygen gas solid carbon water vapour. For example both of the following chemical equations represent the incomplete combustion of heptane a hydrocarbon in gasoline. 808 Explain why the incomplete combustion of hydrocarbons can produce carbon and carbon monoxide.