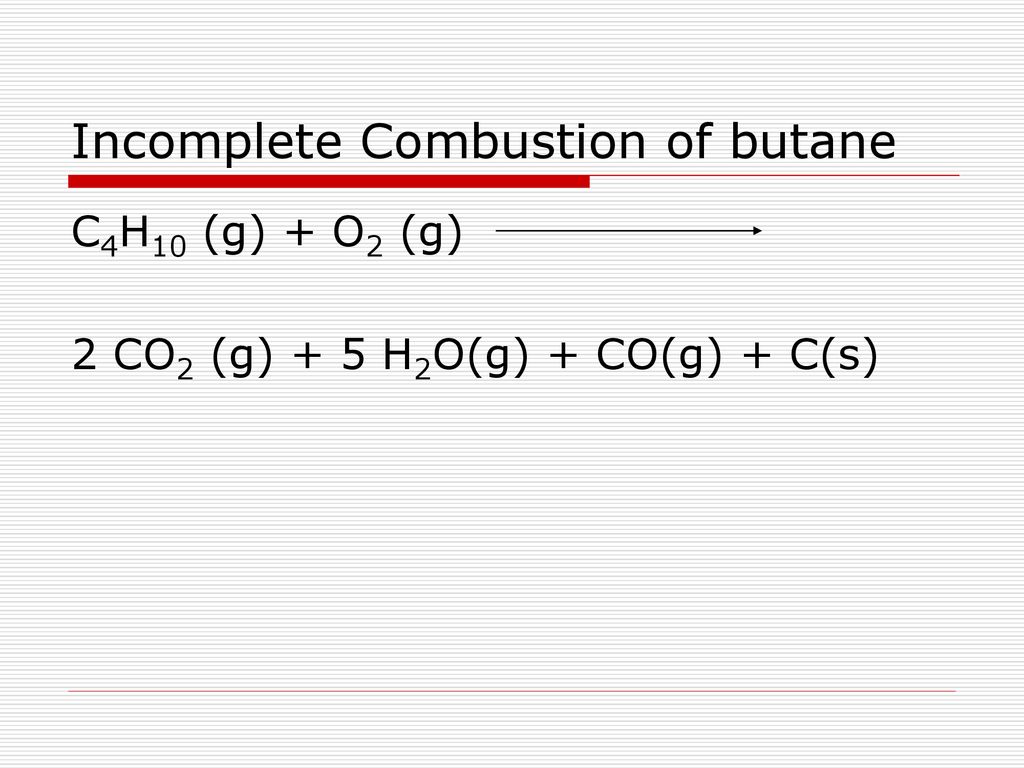
Neat Incomplete Combustion Equation Of Butane
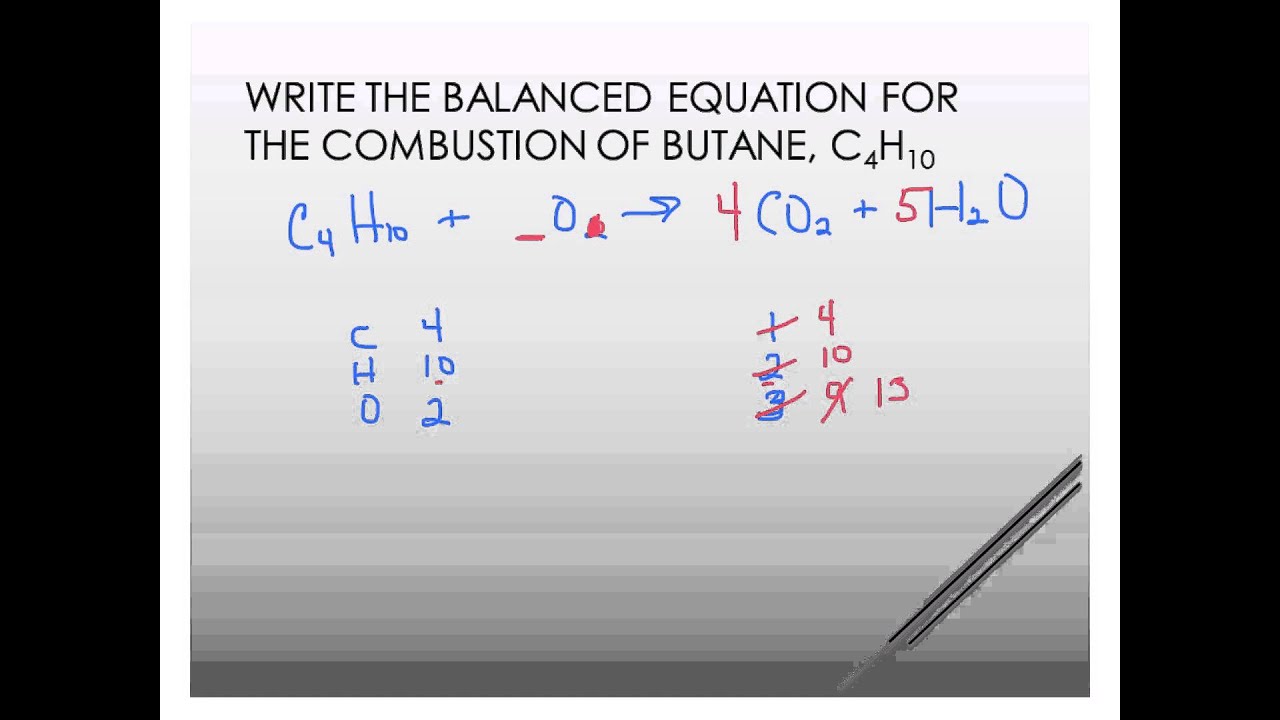
Balancing the Equation Balancing the equation takes some work.
Incomplete combustion equation of butane. Water as a product is obtained in complete combustion as well. A complete combustion of butane b complete combustion of ethanol c incomplete combustion of cyclopentane Cracking Write a balanced molecular equation showing the formation of ethene from the cracking of hexane. Whenever it is said complete combustion what must be remembered is just to write C O 2 instead of C O this is for incomplete combustion Now write coefficients.
An equation for the incomplete combustion of butane in oxygen is. Découvrez les autres cours offerts par Maxicours. In general for incomplete.
I do this kind of problem by going element by element left to right and realizing that if I cant have an odd number of atoms of one element and an even number of atoms of that same element on the other side. The right side of the atom in contrast has two oxygen atoms one carbon atom and one hydrogen atom. Explains TWO of the effects of combustion of fuels.
C4H10 5O2 5H2O 2CO2 CO C Butane Oxygen Water Carbon Dioxide Carbon Monoxide Carbon. Les conditions dune combustion incomplète d. In the commercial production of sugar sucrose the product crystals are washed and centrifuged to partial dryness.
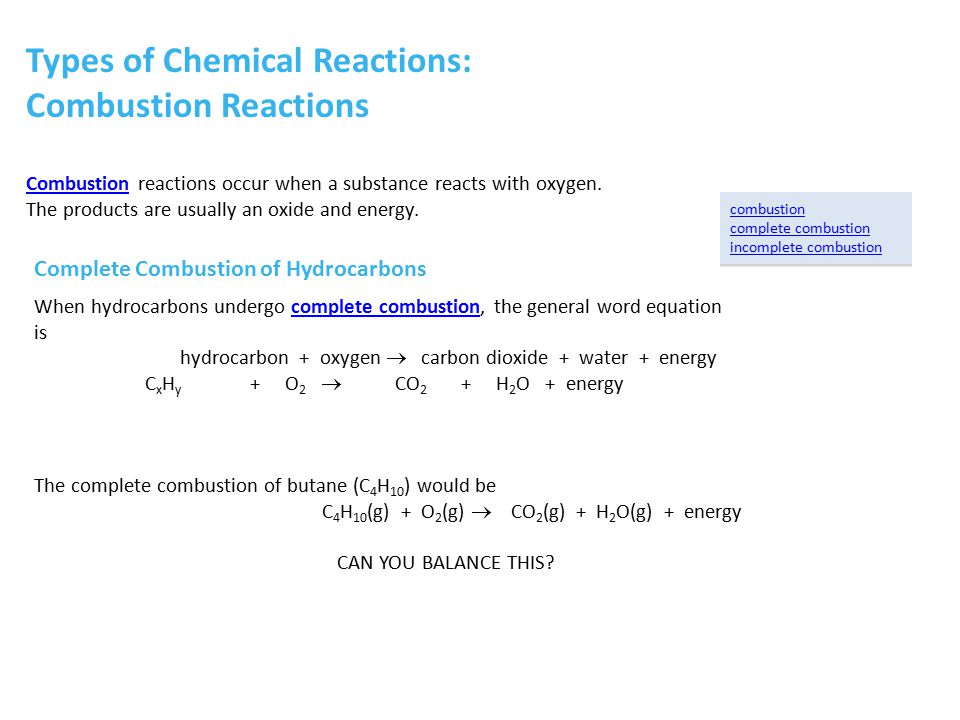
Complete combustion given sufficient oxygen of any hydrocarbon produces carbon dioxide and water. In its unbalanced form the equation for the combustion of butane translates to. C 4 H 1 0 2 1 3 O 2 4 C O 2 5 H 2 O or we can multiply each side by 2.
Complete combustion does NOT give carbon monoxide or sootCheck me out. On the left side of the equation are 10 hydrogen atoms two oxygen atoms and four carbon atoms. The questions posted on the site are solely user generated Doubtnut has no ownership or control over the nature and content of those questions.