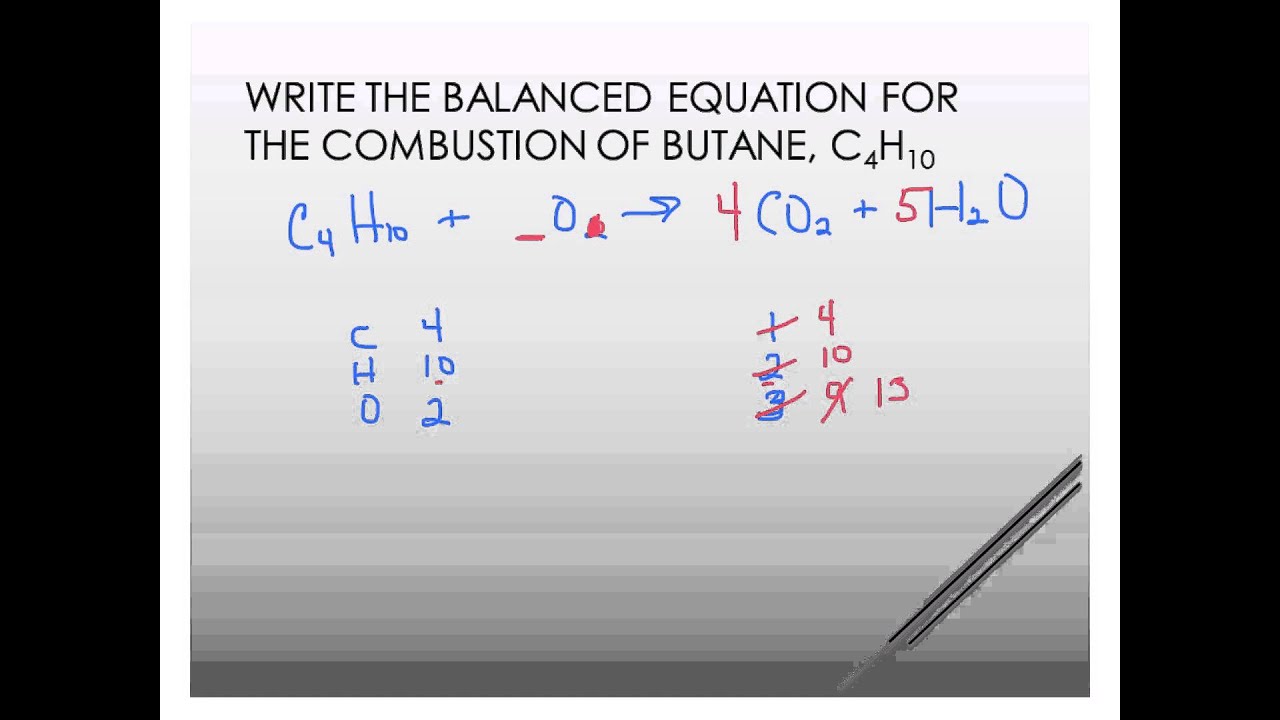
Nice Incomplete Combustion Of Butane Balanced Equation

I do this kind of problem by going element by element left to right and realizing that if I cant have an odd number of atoms of one element and an even number of atoms of that same element on the other side.
Incomplete combustion of butane balanced equation. C4H10 5O2 5H2O 2CO2 CO C Butane Oxygen Water Carbon Dioxide Carbon Monoxide Carbon. Balance the following equation the oxidation of glucose by selecting the correct dropdown numbers for the coefficients. The products of the incomplete combustion of octane C8H18 are carbon monoxide CO and water.
1 Combustion Reactions Write balanced equations for the. A complete combustion of butane b complete combustion of ethanol c incomplete combustion of cyclopentane Cracking Write a balanced molecular equation showing the formation of ethene from the cracking of hexane. Incomplete combustion due to either rich or lean burns may produce harmful combustion by-products such as carbon monoxide and aldehydes.
The crystals are then sent through a rotary dryer where they are contacted with a hot stream of air that reduces the moisture content from 1. C 4 H 10 O 2 4 CO 2 5 H 2 O Counting the oxygen atoms leads to a slight problem - with 13 on the right-hand side. Butane Combustion With butane C 4 H 10 you can again balance the carbons and hydrogens as you write the equation down.
Explains TWO of the effects of combustion of fuels. Nitrogen is included only to indicate that it is present in the medium with some specific ratio to the oxygen amount well technically it is included to make question look complicated. In the commercial production of sugar sucrose the product crystals are washed and centrifuged to partial dryness.
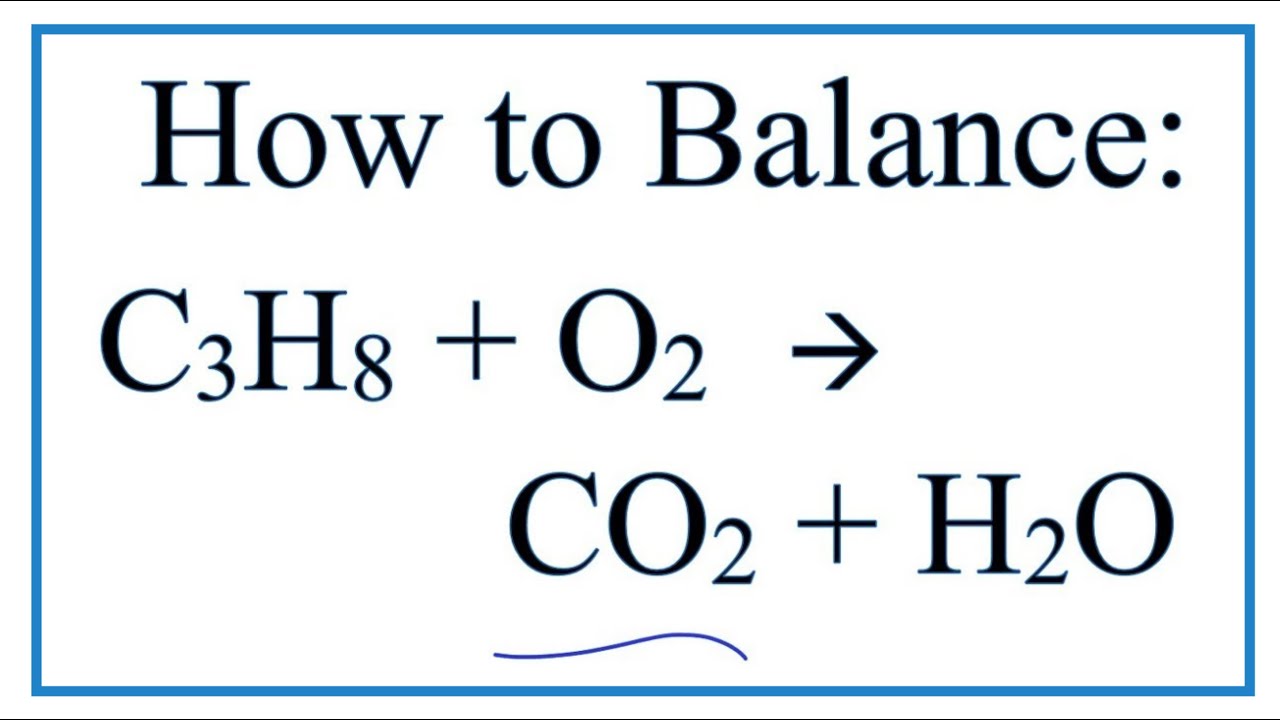
Answered by Abigail P. ONE on each of human health AND the environment makes link between product and effect. 2C3H 8 g 7O2 g 2Cs 2COg 2CO2g 8H2O g 8.
The balanced equation for the combustion of butene is C X 4 H X 8 6 O X 2 4 C O X 2 4 H X 2 O. The equation for incomplete combustion of propane is. The questions posted on the site are solely user generated Doubtnut has no ownership or control over the nature and content of those questions.