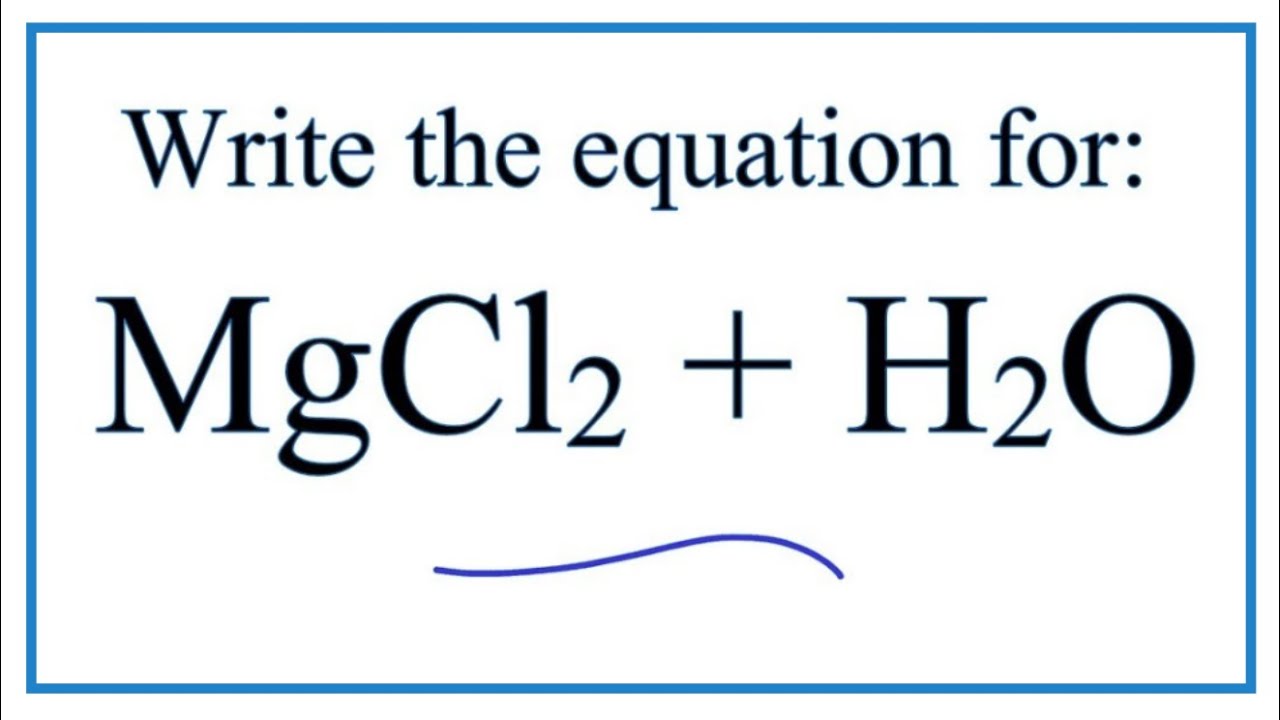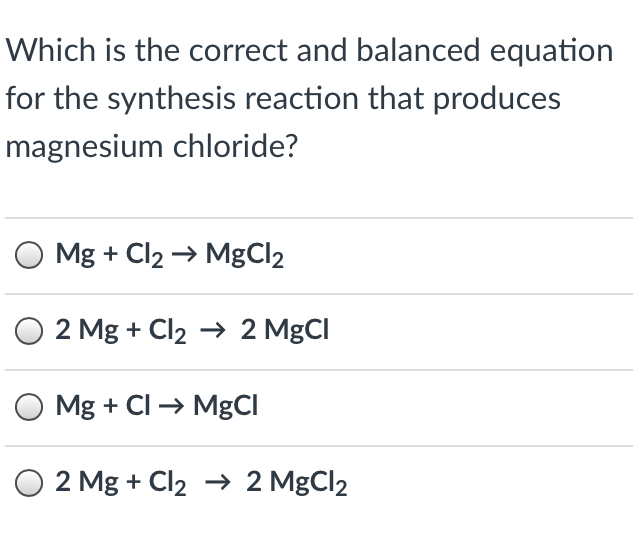Unbelievable Magnesium Chloride Balanced Equation

What type of reaction is Zn HCL.
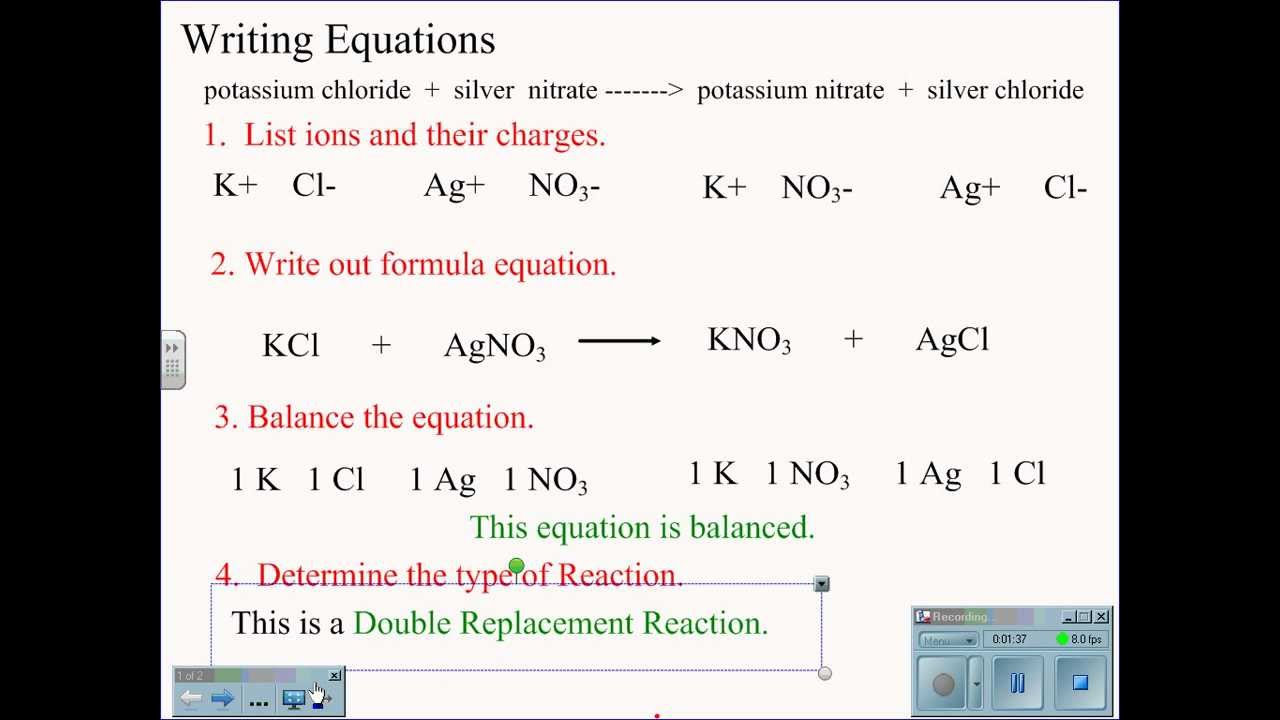
Magnesium chloride balanced equation. Lets write a balanced equation for this reaction. What is the balanced chemical equation for magnesium and hydrochloric acid. Magnesium reacts with hydrochloric acid according to the equation.
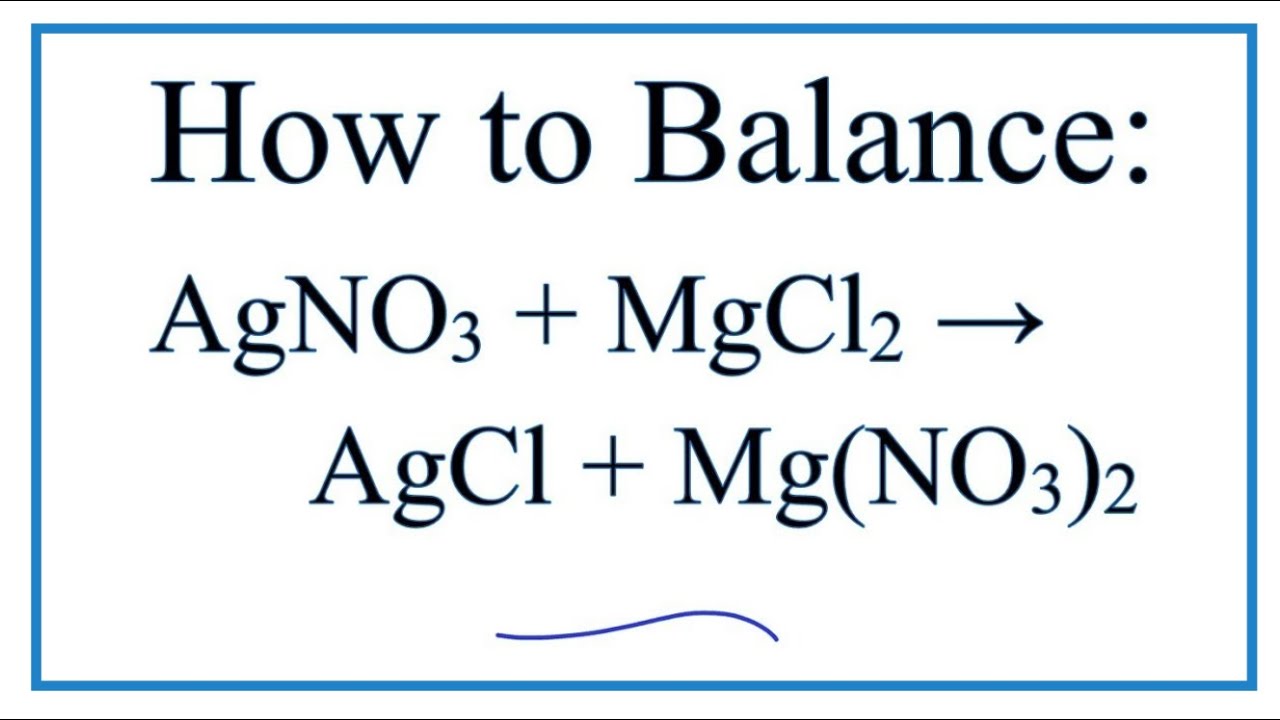
Mg Cl2 -- MgCl2 What mass of magnesium chloride is formed in the reaction between 7 g of Mg and 79 g of Cl2 use the masses to two decimal places on the chart in the. This reaction takes place at. CHEMICAL EQUATIONS II.
The compound is used in medicine as a source of. The chemical reaction between magnesium and hydrochloric acid produces magnesium chloride and hydrogen. Mg s 2 HCl aq -- MgCl2aq H2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas.
A balanced chemical equation is that in which the total number of atoms of each element is equal on both side of the equation. The balanced chemical equation is based on the law of conservation of mass. It is a single replacement reaction where 1 atom of Zinc metal displaces 2 H ions from the hydrochloric acid to form hydrogen gas and zinc chloride.
Mg s 2 HClaq -- MgCl 2aq H 2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the. Magnesium dichloride hexahydrate is a hydrate that is the hexahydrate form of magnesium dichloride. It is a hydrate a magnesium halide and an inorganic chloride.
Magnesium chloride ammonium nitrate magnesium nitrate ammonium chloride MgCl2 2NH4NO3 MgNO32 2NH4Cl mercury II oxide mercury oxygen2HgO 2Hg O2 sodium carbonate calcium hydroxide sodium hydroxide calcium carbonate. Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen. Magnesium reacts with hydrochloric acid according to the equation.