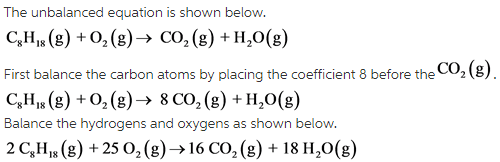Brilliant Octane Plus Oxygen Balanced Equation

The complete combustion of octane C8H18 yields carbon dioxide and water.
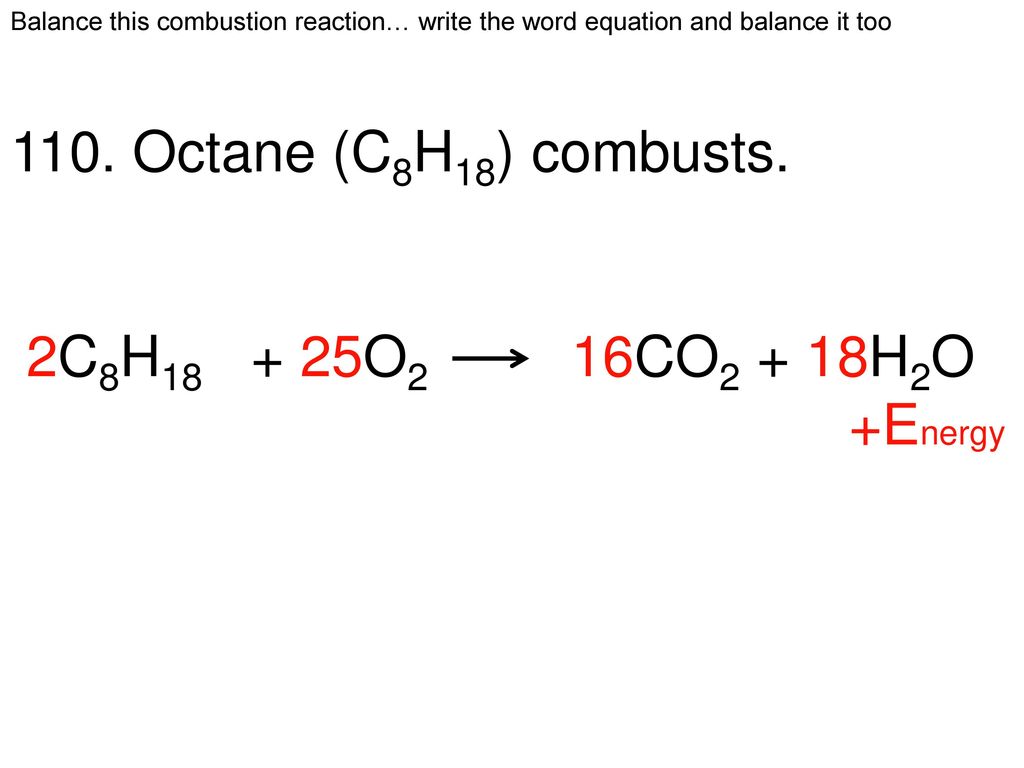
Octane plus oxygen balanced equation. The combustion reaction of octane is2 C8H18 25 O2 16 CO2 18 H2OSo the number of oxygen molecules is 25The isomer trimethylpentane is used as standard in octane. Figure 1 Like Most Hydrocarbons Octane Reacts With Oxygen Gas To Produce Carbon Dioxide And Water. Thus we can balance the oxygen atoms by putting a prefix of 252 on the left side.
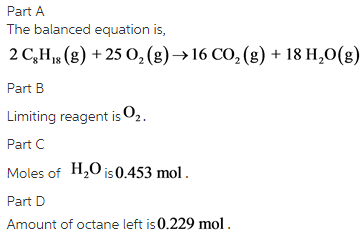
What is the balanced equationLiquid octane reacts with oxygen gas. The balanced equation will appear above. The balanced chemical reaction is 2C8H 18 25O2 16CO2 18H 2O Heres a nice diagram of this reaction Ive found on a French forum.
Endgroup Ed V Jul 18 at 1537. Use uppercase for the first character in the element and lowercase for the second character. This is the combustion reaction for octane C8H 18 a volatile and very flammable alkane that is a component of gasoline.
We now have 4 O atoms on both sides. Octane C 8 H 18 reacts with oxygen to make carbon dioxide and water. Unit Chemical Equations and Reactions Balancing Equations.
Is Octane a liquid. The molecular formula of octane is C8H18. Thus the combustion of octane is given by - C8H18 O2 --- CO2 H2O.
The combustion of a hydrocarbon produces carbon dioxide and water. What is the coefficient of oxygen b in the balanced equation for the complete combustion of octane. CH4 2O2 CO2 2H2O.