First Class Propane Balanced Combustion Equation
Question 13 01 pts The balanced thermochemical equation for the combustion of exactly one mole of propane is.
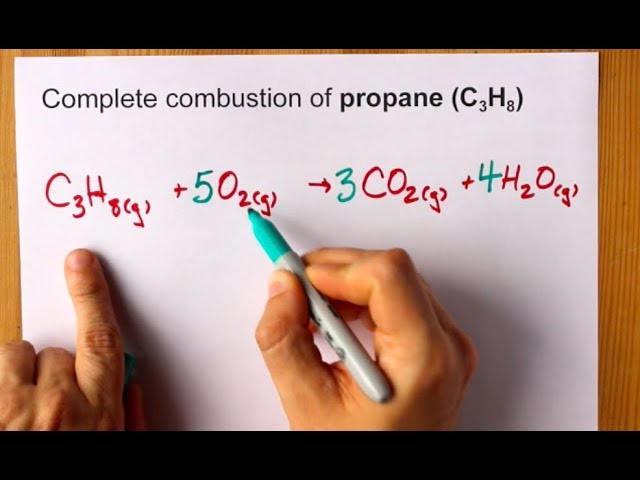
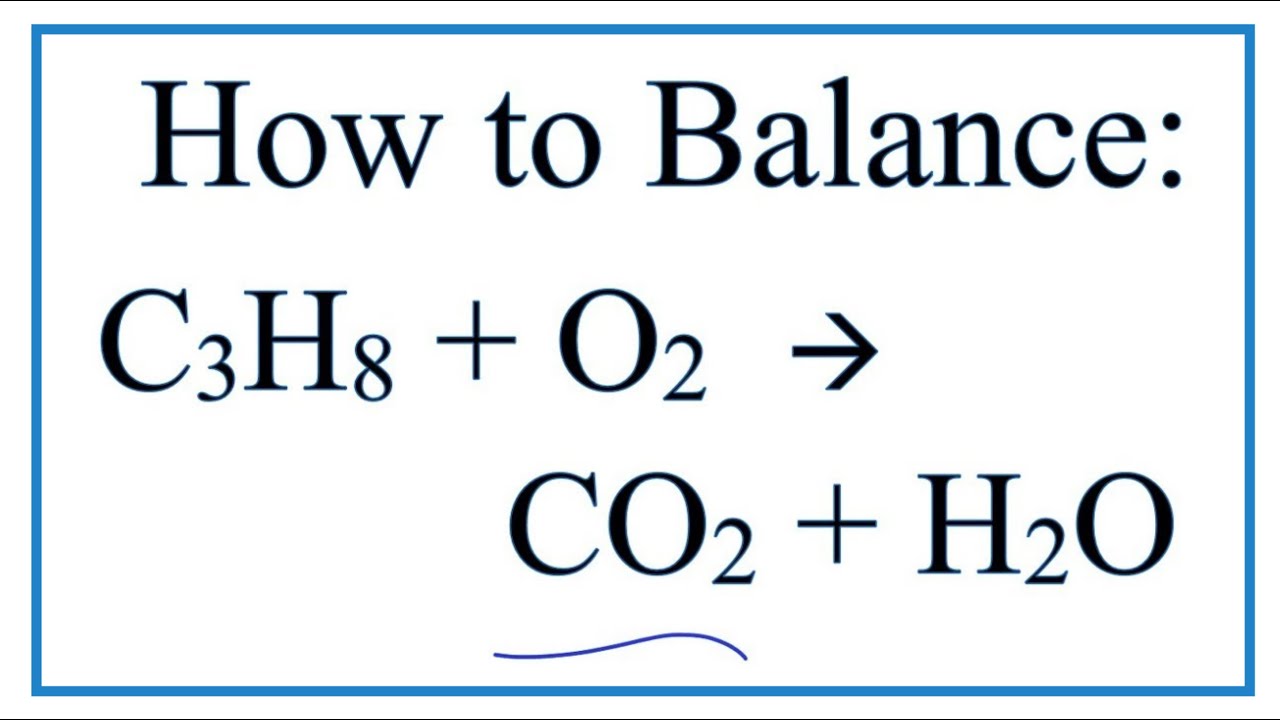
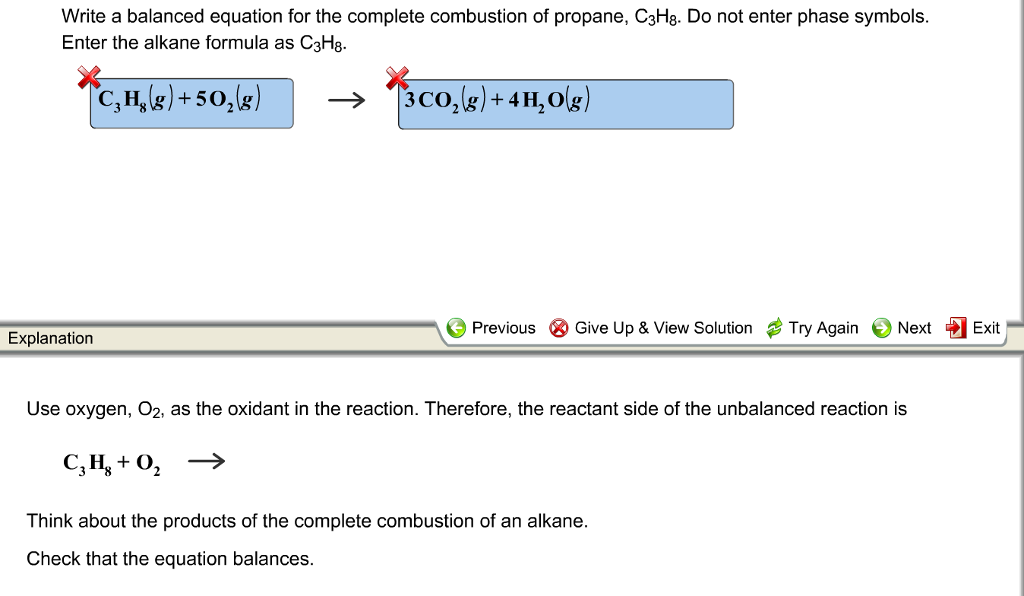
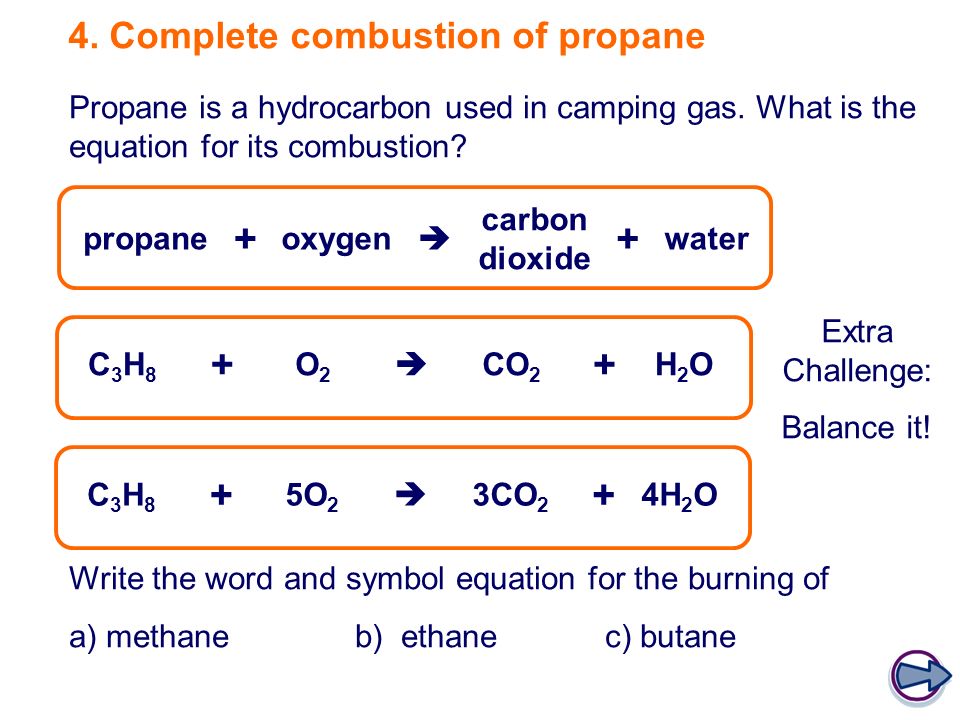
Propane balanced combustion equation. The balanced equation for combustion of propane is. The fuel can be almost anything including methane CH4 propane C3H8 butane C4H10 octane C8H18 or sugar C6H12O6. Write a balanced equation for the combustion of gaseous propane C3H8 a minority component of natural gas in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water.
Propane is a saturated hydrocarbon molecule. To balance atoms place a coefficient 3 before because 3 atoms on the left side while one atom on the right sides. Fuel O2 -CO2 H2O The coefficients of the balanced equation will change depending on the fuel.
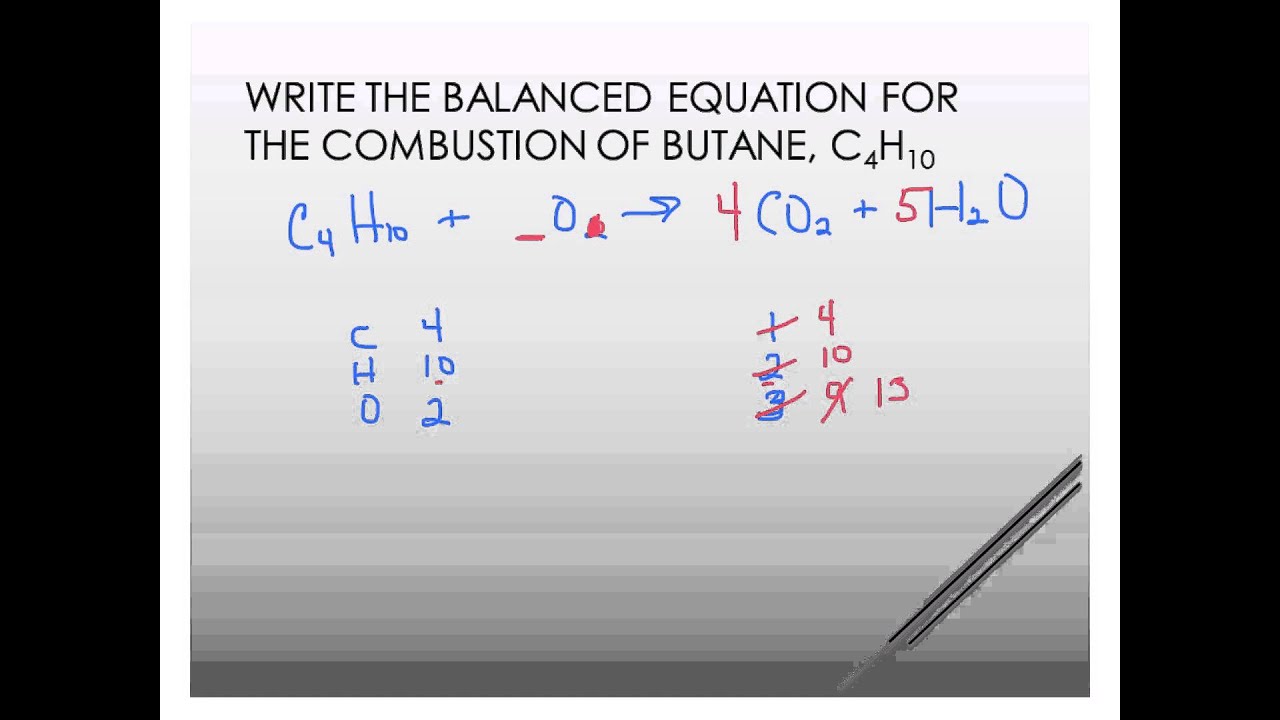
The incomplete combustion of propane C 3 H 8 produces two possible balanced equations. This is a perfect example of a combustion reaction because we have a carbon based compound reaction with oxygen gas to produce carbon dioxide and water. Write and balance equation for the complete combustion of decane C10H22.
Propane undergoes combustion reactions in a similar fashion to other alkanes. I answered C3H8 5O2 3CO2. C 3 H 8 3 O 2 2CO C 4H 2 O C 3 H 8 25 O 2 CO 2C 4H 2 O b Incomplete Combustion Equation -.
In this video well balance the equation C3H8 O2 CO2 H2O and provide the correct coefficients for each compoundTo balance C3H8 O2 CO2 H2O youll. Incomplete combustion produces carbon monoxide which is a poisonous gas. C3H8 g 5 O2g 3 CO2g 4H2O1 AH -2220 kJmol What is the enthalpy change for the reaction.
It is an alkane molecule having 3 carbon atoms in its chain. Complete combustion of propane produces about 50 MJkg of heat. Explained Process of Combustion of Propane.