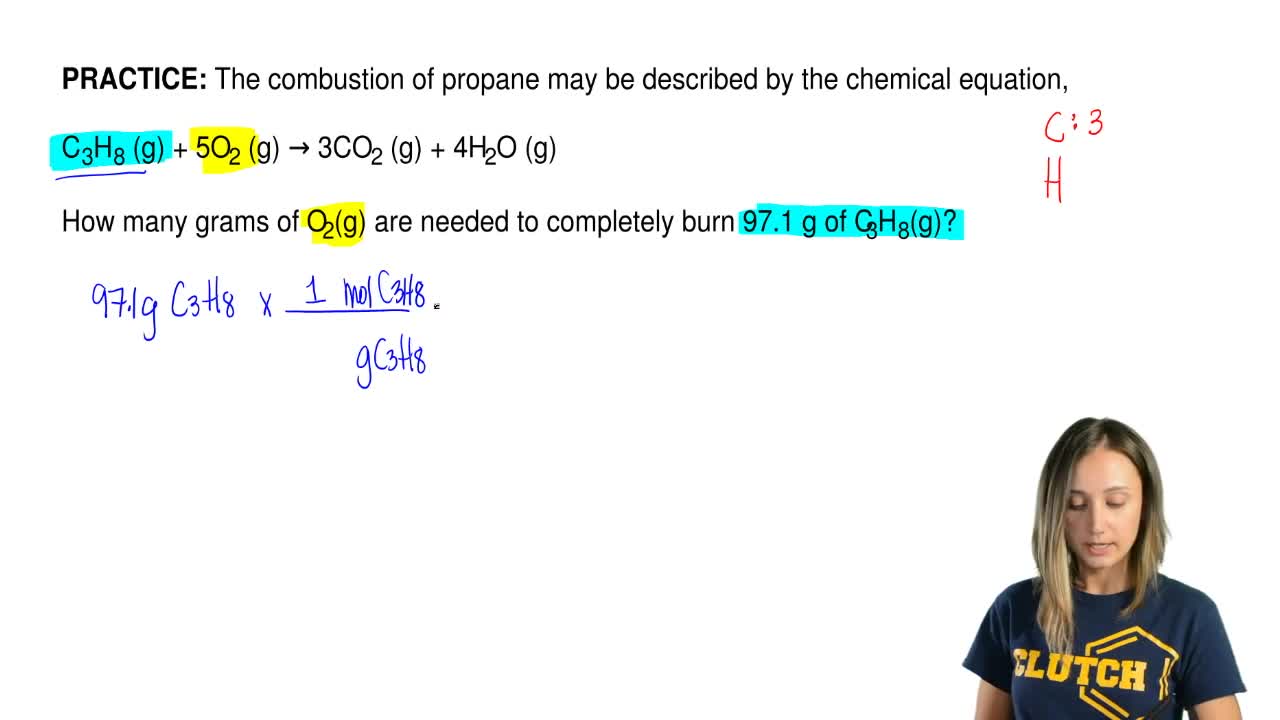Beautiful Work Propane Combustion Equation
The pro- prefix tells us we have 3 carbon.
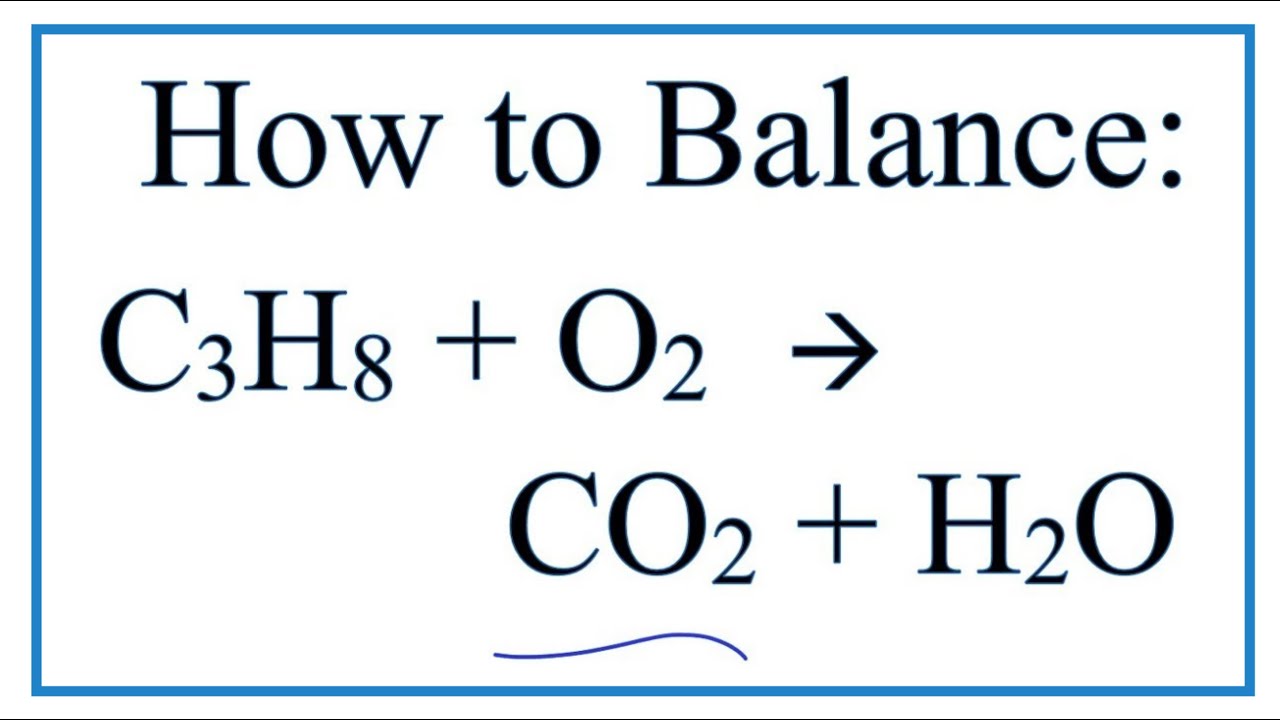
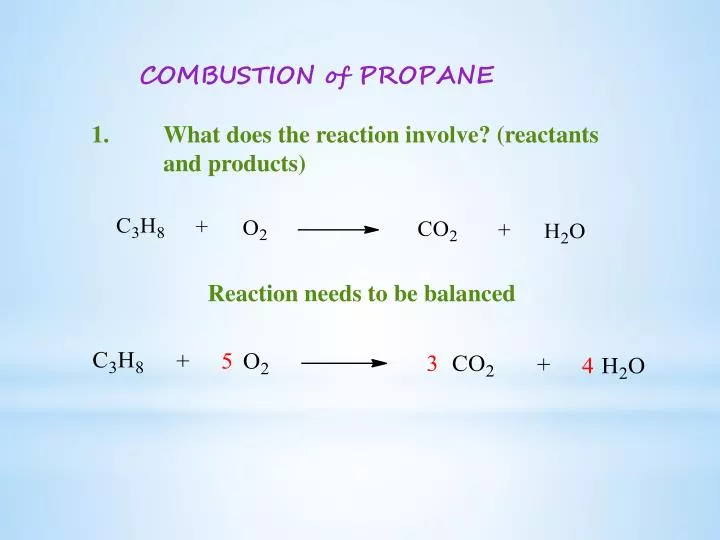
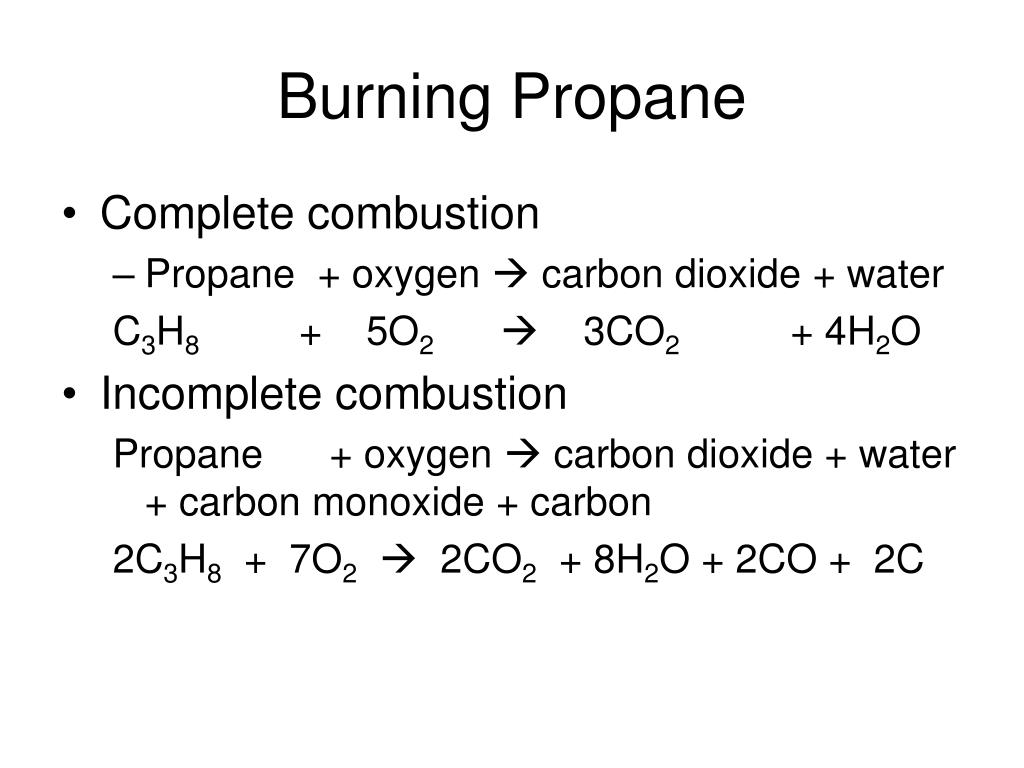
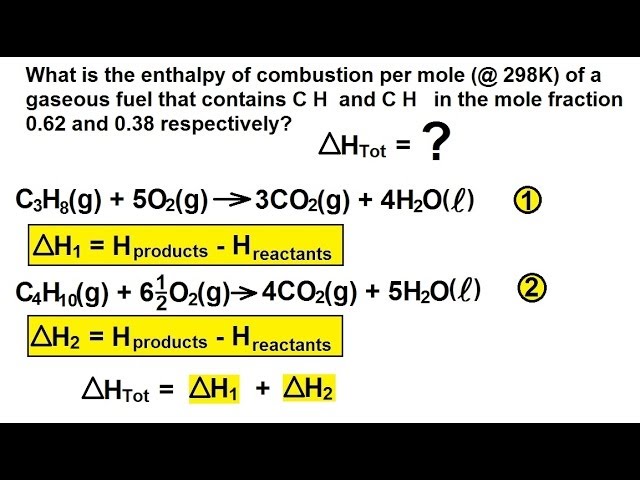
Propane combustion equation. Complete combustion does NOT give carbon monoxide or sootCheck me out. Solved Problem 111 - In this problem we wish to develop the combustion equation and determine the air-fuel ratio for the complete combustion of n-Butane C 4 H 10 with a theoretical air and b 50 excess air. In the presence of excess oxygen propane burns to form water and carbon dioxide.
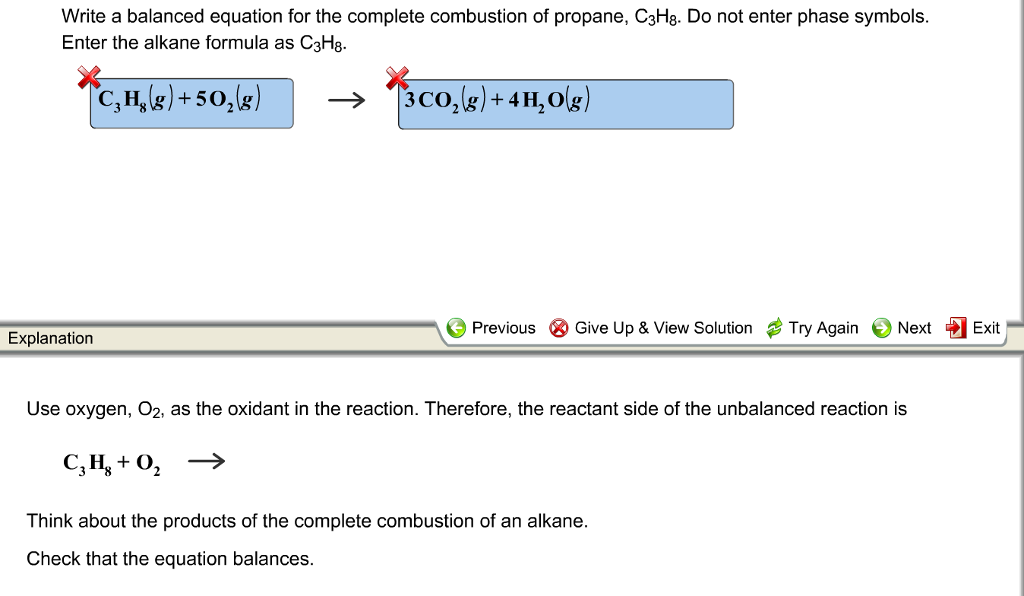
Chemical Engineering questions and answers. The easiest way to identify a combustion reaction is that the products always contain carbon dioxide and water. The general equation for a complete combustion reaction is.
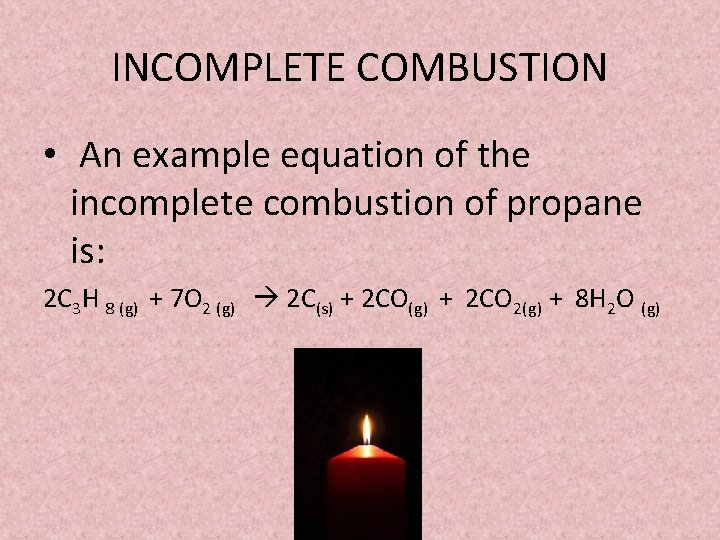
The incomplete combustion of propane C 3 H 8 produces two possible balanced equations. The equation for incomplete combustion of propane is. The chemical equation for stoichiometric combustion of methane - CH 4 - with air can be expressed as CH4 2 O2 376 N2 - CO2 2 H2O 752 N2 If more air is supplied some of the air will not be involved in the reaction.
Complete combustion does NOT give carbon monoxid. Complete combustion of propane results in the formation of carbon dioxide and water vapour. If not enough oxygen is present for complete combustion incomplete combustion occurs.
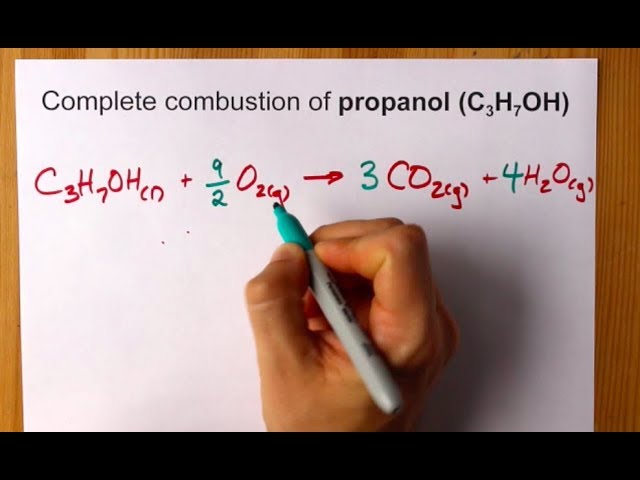
Discovered in 1857 by the. Balancing chemical reaction balancing combustion of propanec3h8o2--co2h2o. Both 1-Propanol C3H7OH and 2-propanol react with oxygen O2 to make carbon dioxide CO2 and water H2O.
C 3 H 8 3. Oxygen is essential for combustion and is used with ethyne acetylene in high-temperature oxyacetylene welding and cutting torches. PROPANE is an energy-rich gas that is related to petroleum and natural gas.