Unbelievable Ch4 Incomplete Combustion

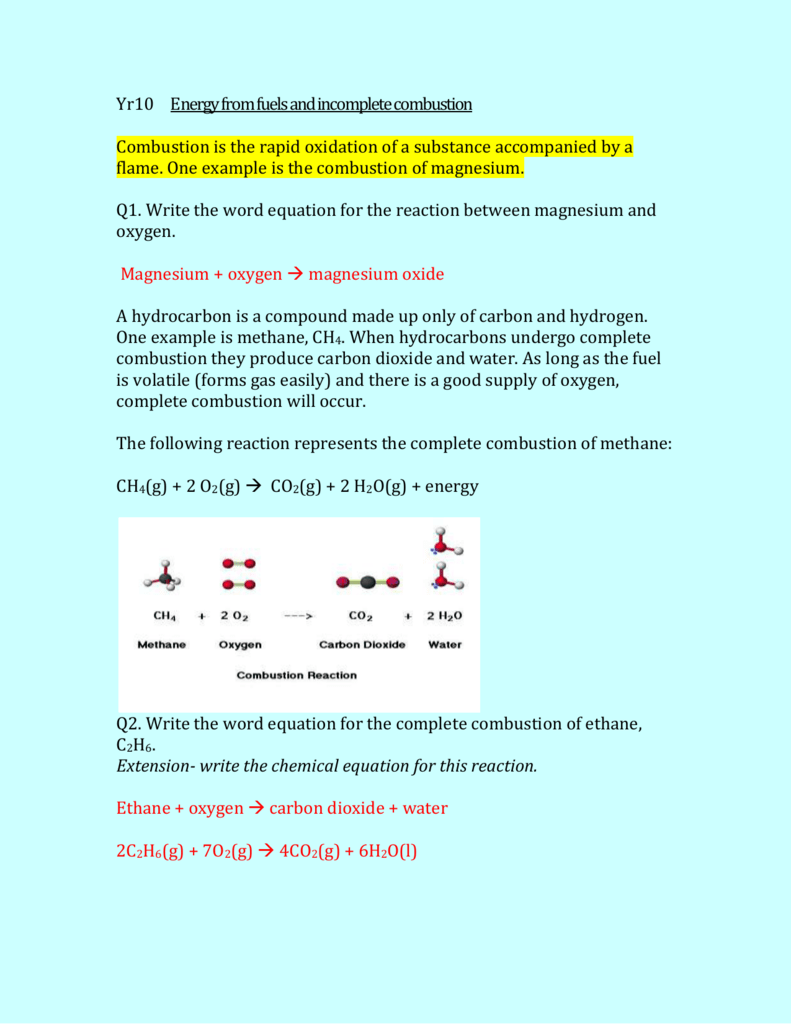
Hydrocarbons react with oxygen to form carbon dioxide and water as the products.
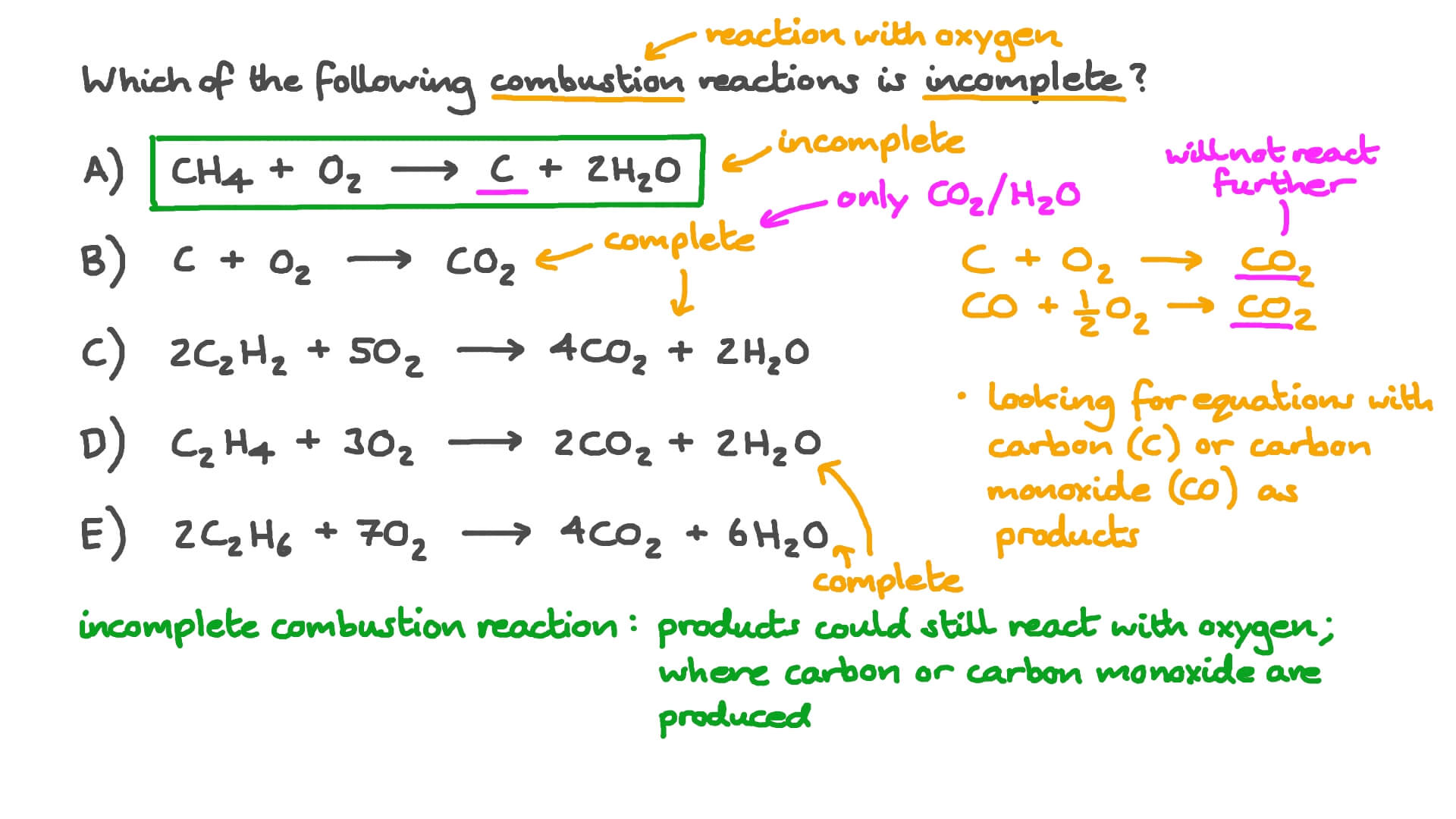
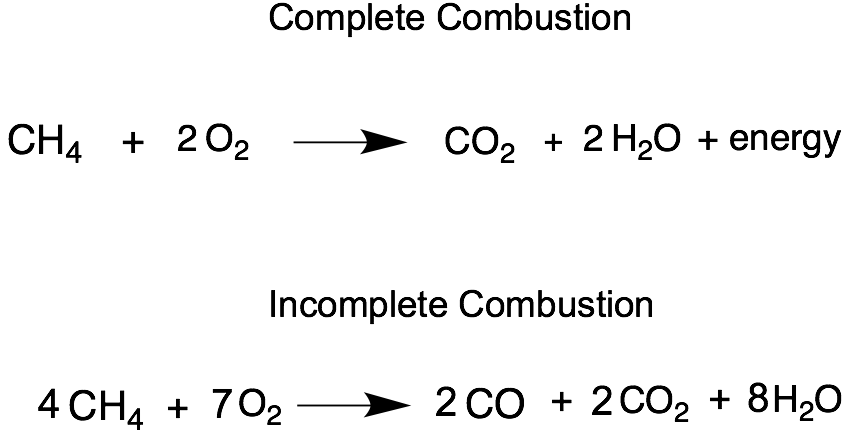
Ch4 incomplete combustion. CH4 O2 ----- CO2 CO H2O Ill leave the equation unbalanced for you to balance as. C H X 4 g O X 2 g C s 2 H X 2 O g. Hence the incomplete combustion forms a number of byproducts.
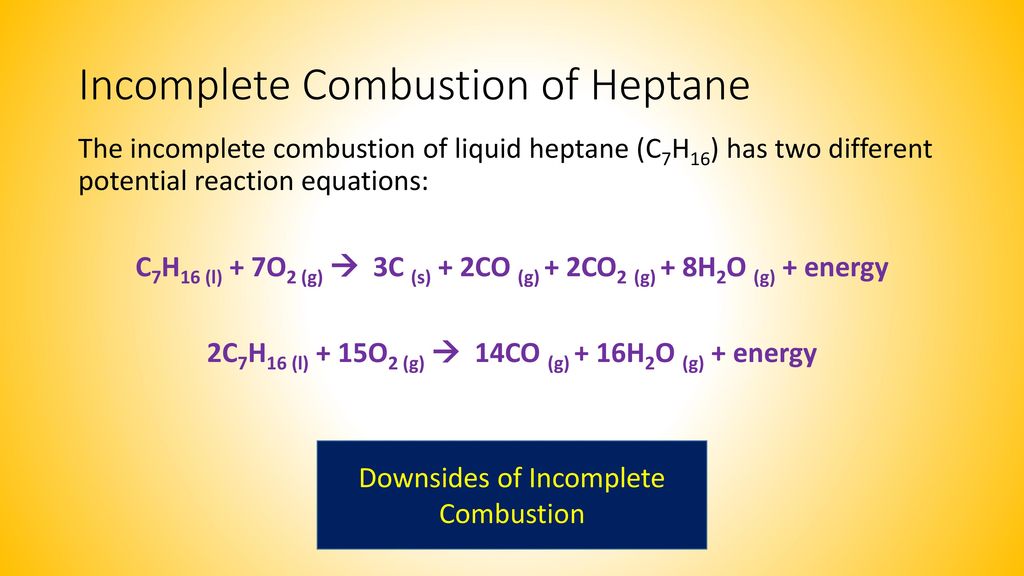
Hydrocarbon oxygen- carbon carbon monoxide water. Not converted to CO2 results in CH4 CO andor VOC emissions and is due to incomplete combustion. The combustion of methane is represented by the equation.
The Inc omplete Combustion of Natural Gas - Methane. CH4 O2 CO2 H2O Unfortunately this equation is incomplete. Occurs when there is a good supply of oxygen.
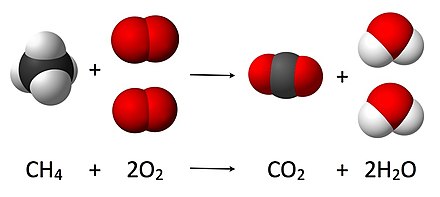
In this video well balance the equation CH4 O2 CO2 H2O and provide the correct coefficients for each compoundIn order to balance CH4 O2 CO2 H. In many cases these substances can be toxic. Howeverthe oxidation of methane to soot is generally given as the following.
It can be detected by. Formation of N2O during the combustion. What is the balanced form of the equation ch4 o2 co2 h2o.
CH4g 2O2g CO2g 2H2Og A common misconception is that carbon monoxide is the only product of the incomplete combustion of a hydrocarbon. What quantities and values would be needed to calculate the theoretical amount of oxygen. Soot hydrogen and nitrogen oxides are other common byproducts of incomplete combustion.