Ace Coefficient In Chemical Equation
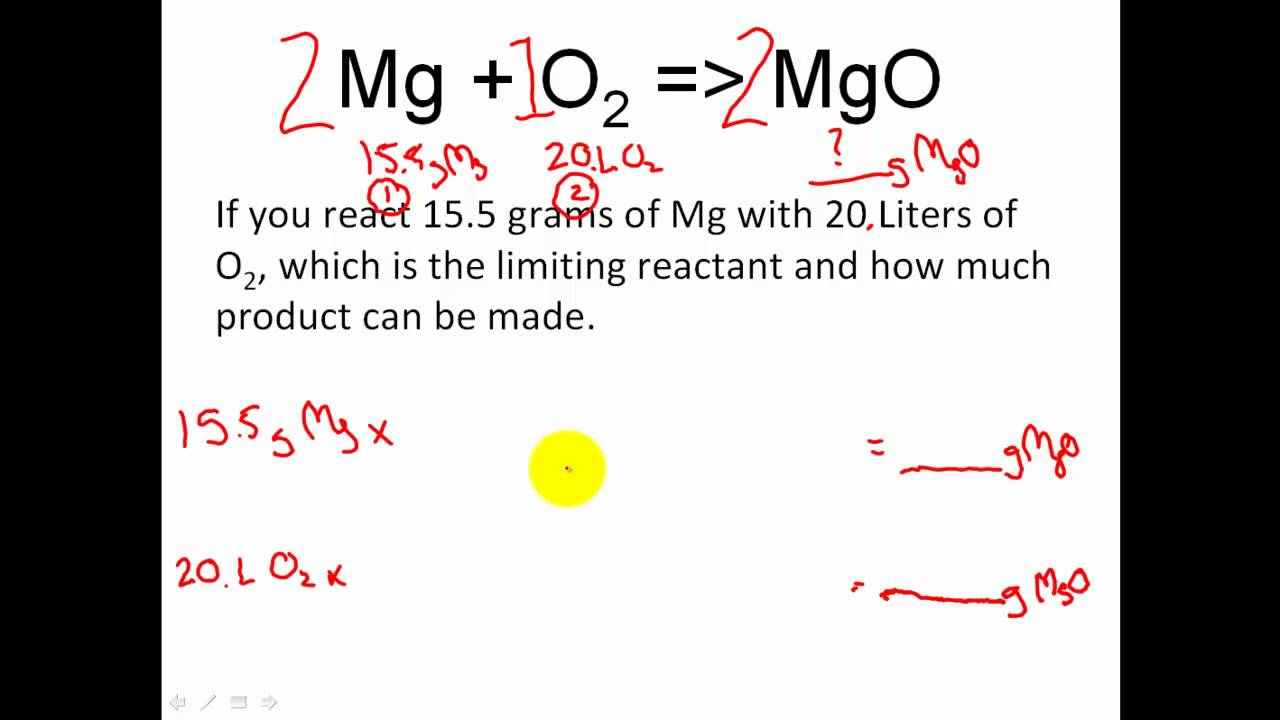
Coefficients number in front of a chemical formula indicate moles of a compound.
Coefficient in chemical equation. Heres something important to remember about. It shows how many atoms or molecules of the substance are involved in the reaction. When no coefficient is written in front of a species the coefficient is assumed to be 1.
Coefficients are used to balance chemical equations. This amount can represent either the relative number of molecules or the relative number of moles described below. The 2 written before the symbol Hg is called a coefficient.
A coefficient is a whole number multiplier. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers. Use uppercase for the first character in the element and lowercase for the second character.
Fe Au Co Br C O N F. What do coefficients represent in a chemical equation. Ionic charges are not yet supported and will be ignored.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. Coefficients are used in all chemical equations to show the relative amounts of each substance present. It shows how many atoms or molecules of the substance are involved in the reaction.
What do coefficients in a chemical equation represent macroscopically. A coefficient is a number placed in front of a chemical symbol or formula. 2H2 O2--- 2H2O Note the presence of a two in front of the hydrogen and also the water.













