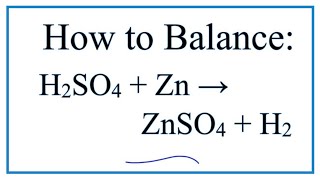
Nice Zn + Sulphuric Acid

This is a double replacement reaction where does Zn up replaces the H in the sulfuric acid.
Zn + sulphuric acid. Zinc reacts with dilute sulphuric acid to produce hydrogen gas H 2 and zinc sulphate. Zn 2H2SO4 hotconcentrated ZnSO4 SO2 2H2O The acid is reduced to sulphur dioxide by zinc. It is a conjugate acid of a hydrogensulfate.
Zinc react with sulfuric acid Zn H 2 SO 4 ZnSO 4 H 2 Check the balance Zinc react with sulfuric acid to produce zinc sulfate and hydrogen. Sulfuric acid is a sulfur oxoacid that consists of two oxo and two hydroxy groups joined covalently to a central sulfur atom. Zhu Yugang answered The Balanced Equation For Zinc Nitrate And Sulfuric Acid is Zn NO32H2SO4ZnSO42HNO3.
The reaction of zinc dissolution in sulphuric acid solution can be represented by the equation 1. Zn 2H Zn2 H 2 2 If these two reactions are observed separately in the process of corrosion then by the consistent appliance of regularities on which corrosion processes. In aqueous solution the Zn II ion is present as the complex ion Zn H 2 O 6 2.
Zn s H 2 SO 4 g ZnSO 4 s H 2 Zinc dil. The bacteriologist Franz Ziehl 18591926 and the pathologist Friedrich Neelsen 18541898. Zinc sulfate is produced by treating virtually any zinc-containing material metal minerals oxides with sulfuric acid.
Most studies are related to solving practical problems arising from the hydrometallurgical production of zinc and therefore are focused on the investigation of the dissolution of pure ZnO andor of ferrites which are formed during the oxidative roasting of zinc. 25 rows Zn 1 to 40 ppm. Type of Chemical Reaction.
Its a straightforward reaction to balance just be sure to count of the atoms on each side of the equation carefully. Concetrated sulphuric acid is an oxidising agent and so the reaction bewetween the hot acid and zinc is a typical redox reaction. Granulated zinc Znreacts with dil sulphuric acid H2SO4 to produce hydrogen H2gas Granulated zinc is used instead of solid zinc as during the reaction zinc sulphate gets deposited on the surface of Zn which slows down and eventually stops the production of H2 gas.