Neat Write A Balanced Equation For The Combustion Of Ethanol

Ethanol C2H5OH is composed of C H and O.
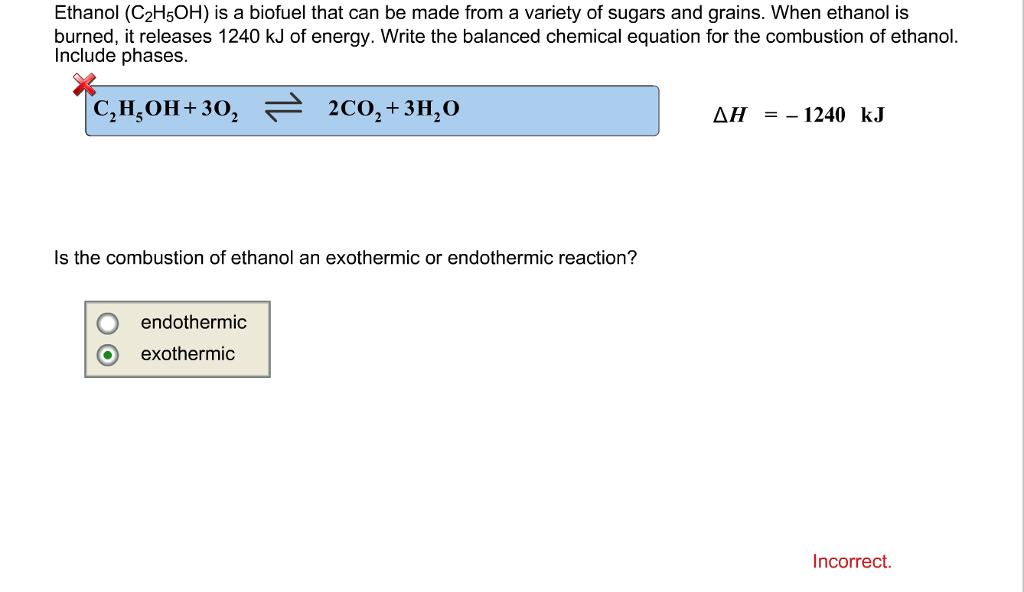
Write a balanced equation for the combustion of ethanol. Write the balanced equation for the combustion of ethanol. Calculate the standard heat of formation of ethanol ΔH. E calculate delta G for the reaction at 100C.
A write a balanced equation for the combustion of 1 mole of ethanol with oxygen gas into carbon dioxide gas and liquid water. We can calculate for the reaction by using Equation 528 and data in Table 53. Chemistry questions and answers.
Place a coefficient of 3 in front of the O2 on the left side. C calculate delta S for the reaction. Using the balanced equation for the combustion of ethanol answer the following question.
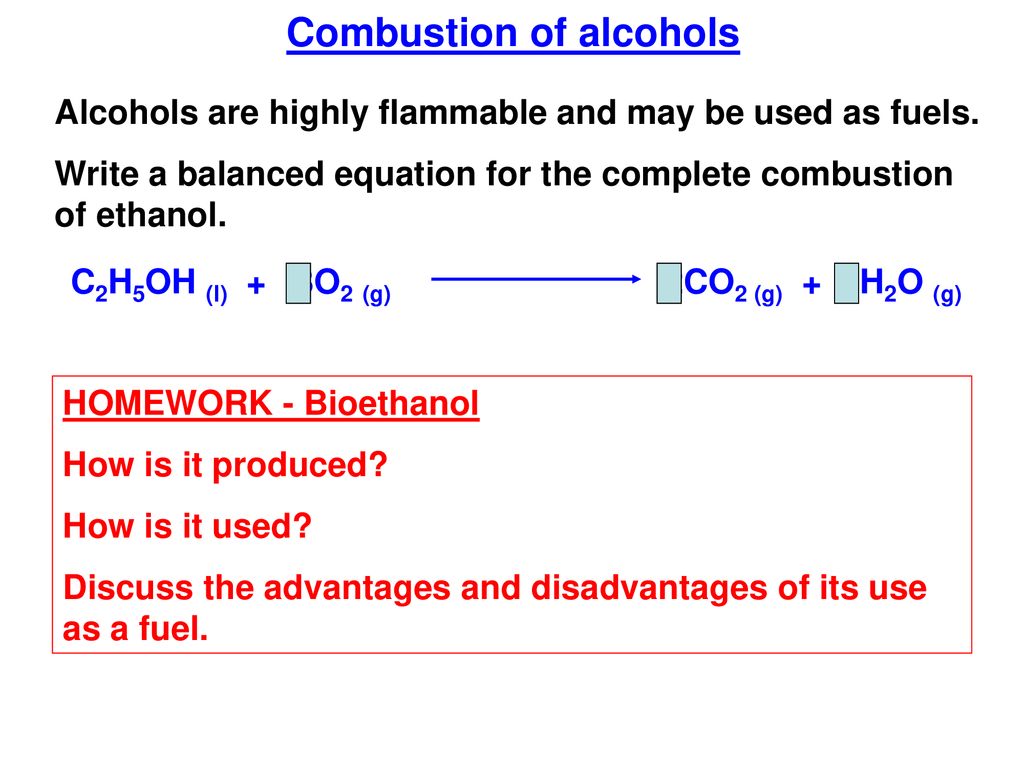
Write a balanced equation for the combustion of ethanol C2H5OH l in oxygen. If n is even divide by 2. 3 To balance a chemical equation enter an equation of a chemical reaction and press the Balance buttonThe formula for ethanol is ce C_2H_5OHA chemical equation is the representation of the chemical reactionsThe formula for heptane is C7H16.
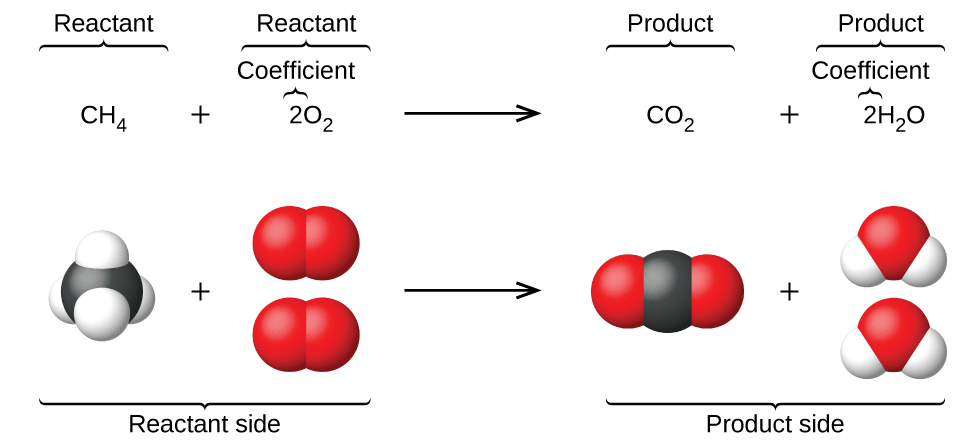
CnH 2n2Ol O2g CO2g H 2Ol. C2H5OH O2 CO2 H2O Since both C and H are appearing at only once place each side they should be balanced. Organic Combustion - kentchemistry com Complete combustion given sufficient oxygen of any hydrocarbon produces carbon dioxide and water Equations It is quite important that you can write properly Multimedia.
The heat of combustion of methanol CH 3 OH as described in the equation CH 3 OH l 1½O 2 g CO 2 g 2H 2 O l is 715 kJ mol 1 and the heats of formation of carbon dioxide gas and water liquid are 3935 kJ mol 1 and -2858 kJ mol 1 respectively. Using the balanced equation for the combustion of ethanol answer the following question. Videos and illustrations from Chapter 6 Lesson 1 of the Middle School Chemistry Unit produced by the American Chemical Society Balance Chemical Equation.