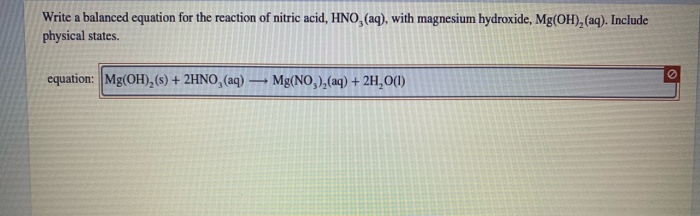Breathtaking Magnesium Hydroxide And Nitric Acid Balanced Equation

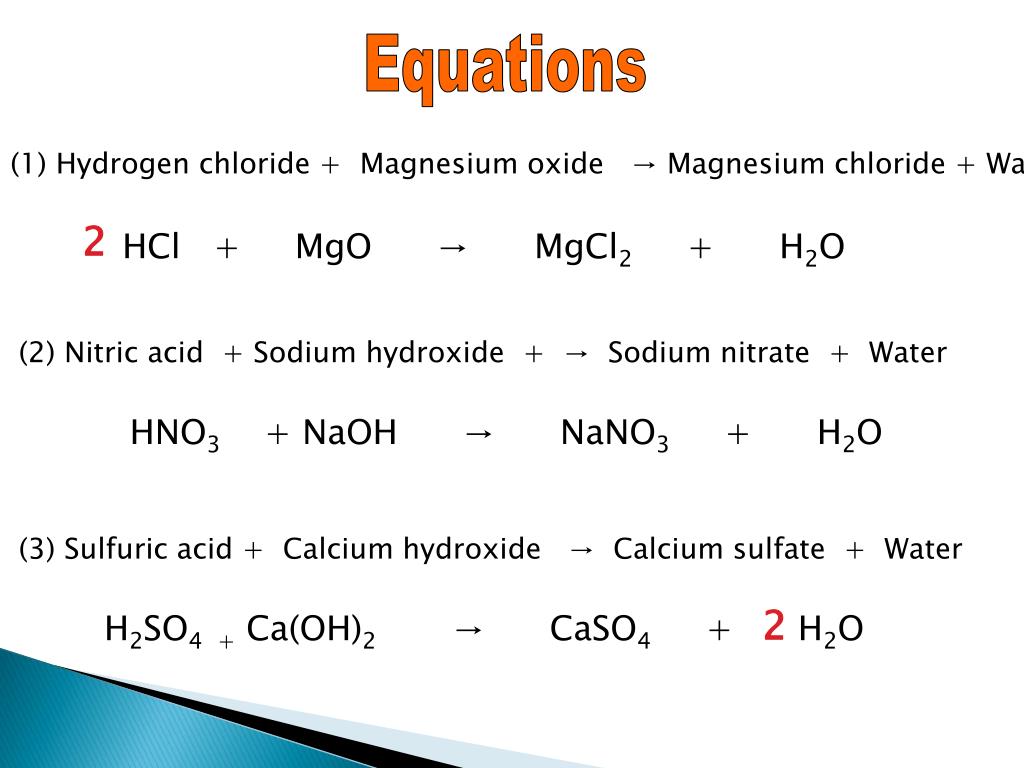
Never change the subscripts the small numbers after elements.
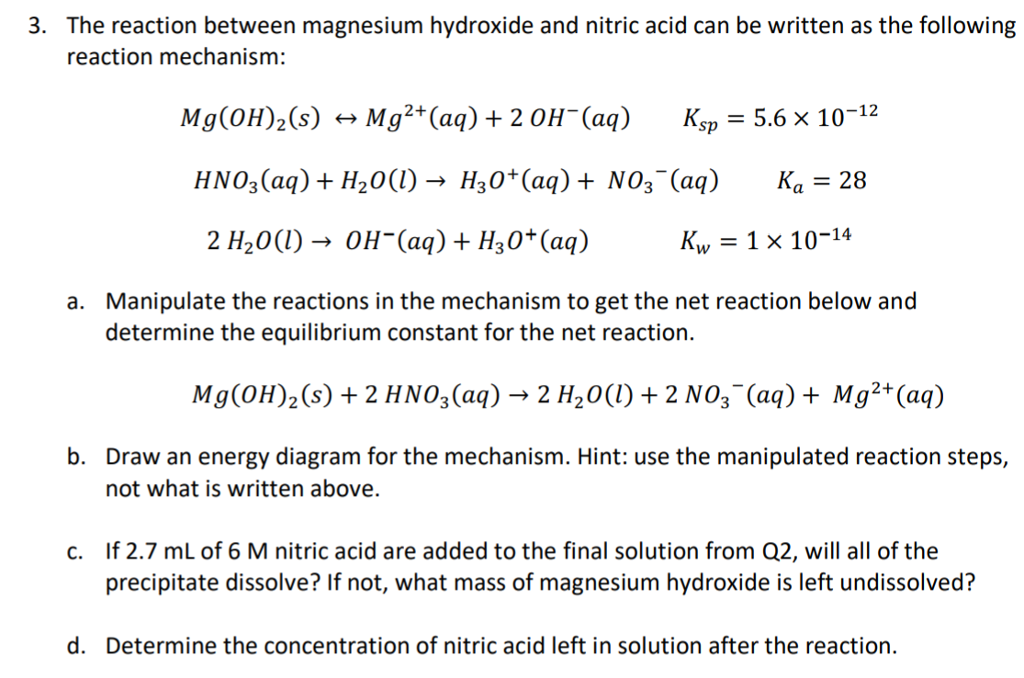
Magnesium hydroxide and nitric acid balanced equation. Lakhmir Singh Chemistry Class 10 Solutions Acids Bases And. Sulfuric acid reacts with sodium hydroxide. Here we have a neutralization reaction.
Asked Sep 8 2018 in Chemistry by PriyaBharti 537k points icse. Acid rain department of chemistry washington. Carbon dioxide is a non-electrolyte water is a weak electrolyte so in the ionic equation they are written in molecular form.
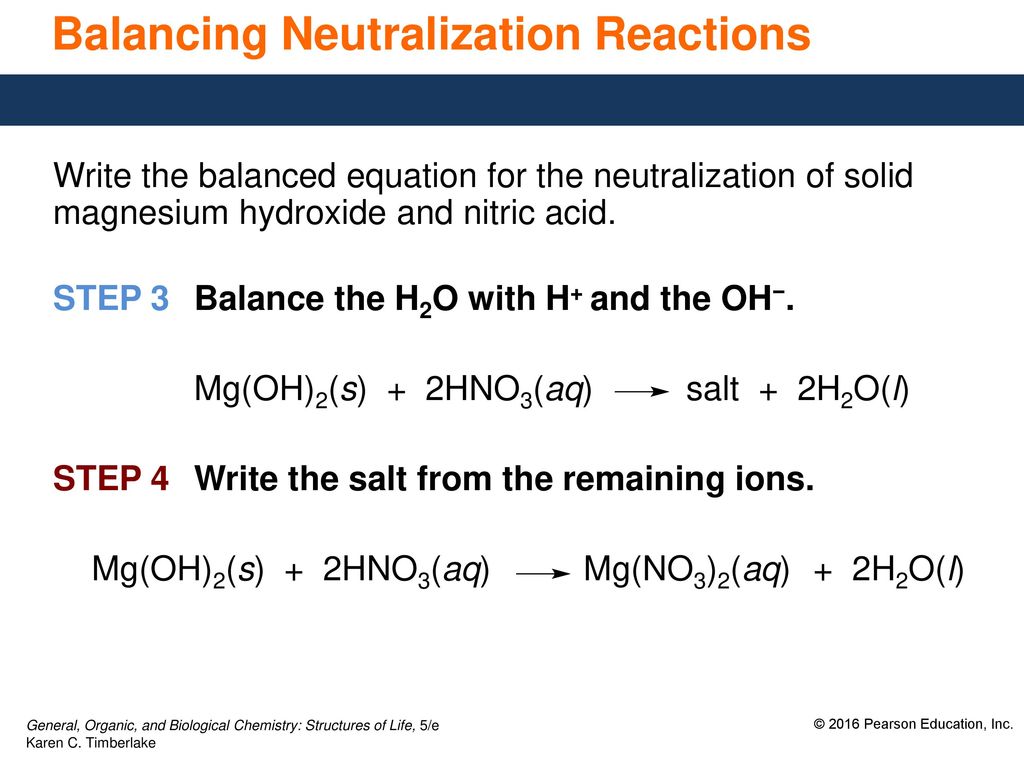
How to Balance Mg OH2 HNO3 Mg NO32 H2O Magnesium hydroxide Nitric acid When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation. What is the conflict of the story of sinigang. H₂O is a weak electrolyte so we write it as a molecule.
For this reaction we have a single displacement reaction. 5 f Distinguish between the â Magnesium metal reacts with hydrochloric acid to form magnesium chloride and hydrogen gas. Balanced Equation for nitric acid and barium hydroxide April 18th 2019 - Balanced Equation for nitric acid and barium hydroxide Submitted by Sunshine on are right but I keep coming up with an extra H amp O b c of the OH on the right side but I don t undestand how to balance.
Thus allowing more solid MgOH2 to dissolve. Who of the proclaimers was married to a little person. Therefore it is an acid-base reaction and the products are magnesium nitrate and water onlychemical equation.
When 2 - methyl -1- butene treated with Hydrogen Bromide then there is addition takes place. Write a balanced molecular equation and a net ionic equation for his reaction Write the balanced molecular complete ionic and net ionic equations for the following reactions in aqueous solution. The salt is the magnesium nitrate.