Heartwarming Naoh Plus Hcl Balanced Equation

To balance net ionic equations we follow these general rules.
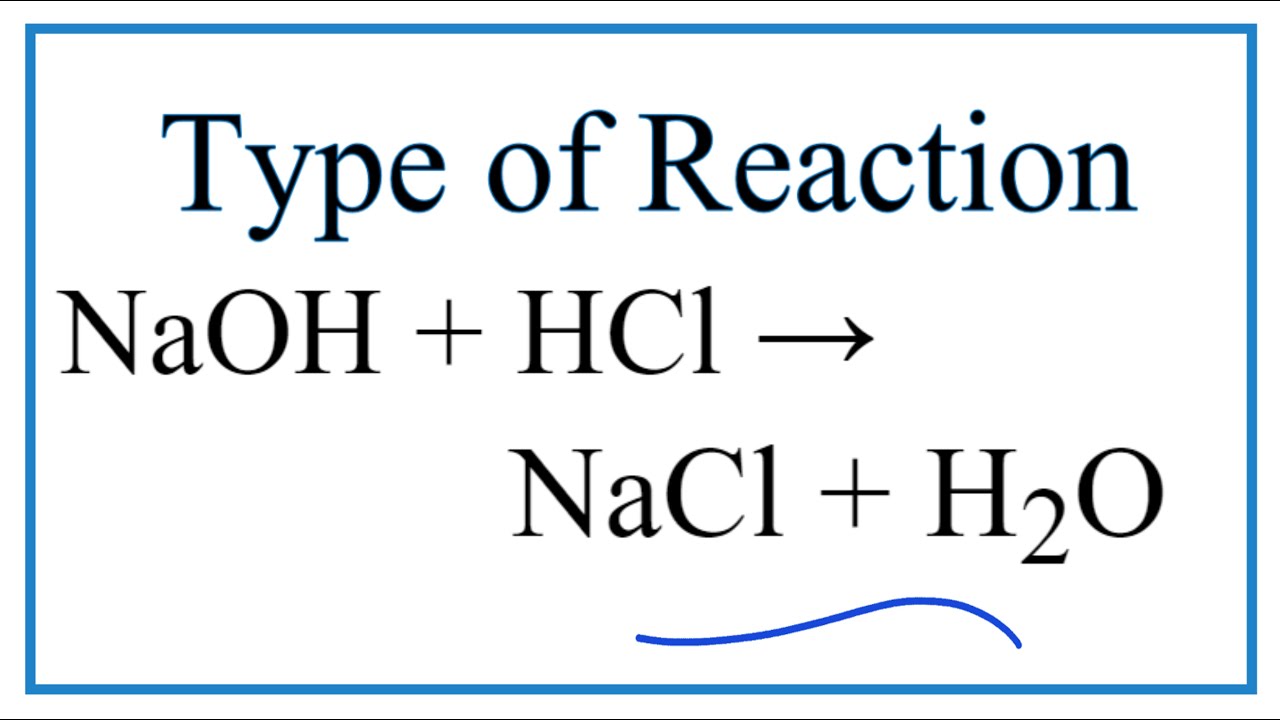
Naoh plus hcl balanced equation. Volume of NaOh despensed mL 1590-00 1590 mL Moles of NaOH concent view the full answer Previous question Next question. Answer - HCl aq NaOH aq NaCl H2O Given - i molar concentration of NaOH 01482 M Titration 1. Sodium Hydroxide and Sulfuric Acid Balanced Equation Now I will balance sulfuric acid and sodium hydroxide reaction.
It may possibly usually be managed with suitable chemical maintenance. Formula Equation For Sodium Hydroxide With Hydrochloric Acid. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2.
The heat exchanged by the reaction qreaction can be used to determine the change in enthalpy of the reaction. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles. If Algae will become a difficulty the pool could have for being drained and washed having a 5050 mixture of muriatic acid and h2o.
Positive hydrogen ions from HCl and negative hydroxide ions from NaOH combine to form water. Write the balanced molecular equation. What is the limiting reactant in this chemical equationIn the neutralization of 10 M HCl and 10 M NaOH NaOH aq HCl aq - NaCl aq H2O.
HClO 4 NaOH NaClO 4 H 2 O is a neutralization reaction also a double displacement reaction. The limiting reagent row will be highlighted in pink. When you balance the equation you.
HCl aq NaOH aq NaCl aq H2O l heat. H₂SO₄ NaOH -----Na₂SO₄ H₂O Step-2In the left side we have H SO₄ Na O To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion. The balanced equation will appear above.