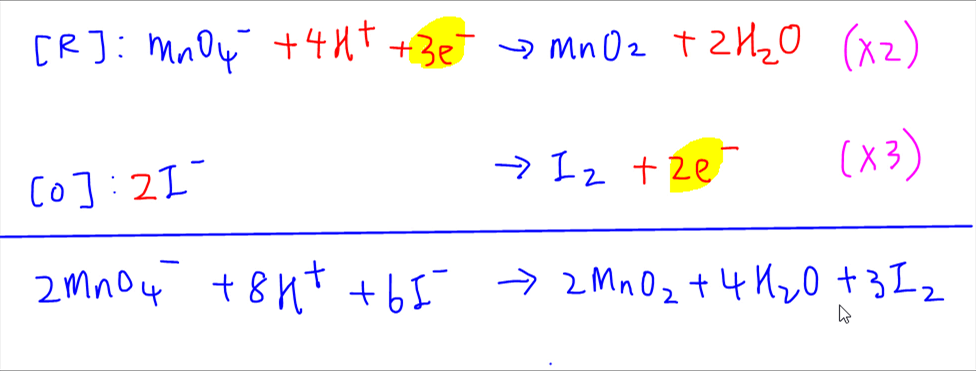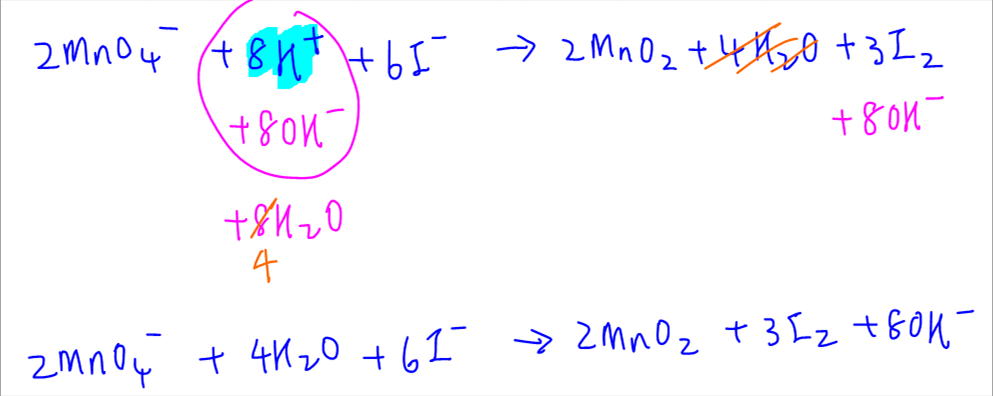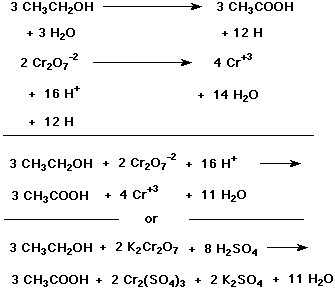Wonderful Redox Equation Balancer

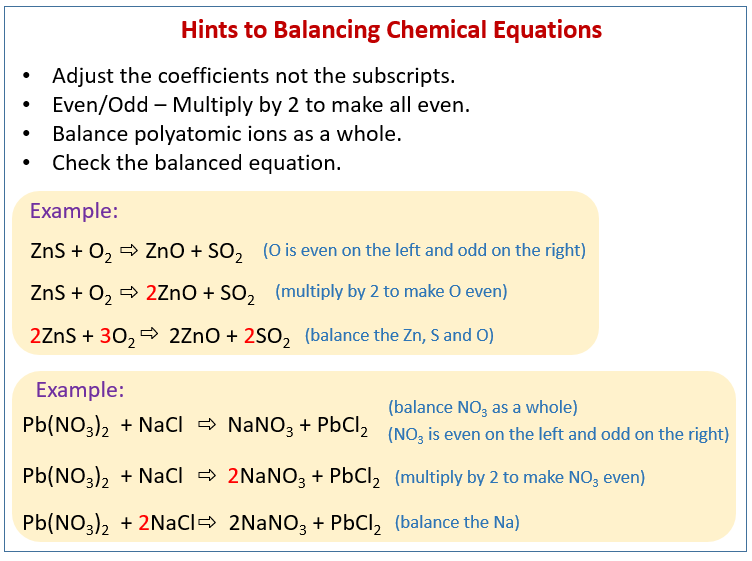
Balance the rest of the atoms Hs and Os using H.
Redox equation balancer. Use this online half reaction method calculator to balance the redox reaction. Balance the unbalanced redox reaction without any complications by using this online balancing redox reactions calculator. The Half-Reaction Method of Balancing Redox Equations A powerful technique for balancing oxidation-reduction equations involves dividing these reactions into separate oxidation and reduction half-reactions.
We then balance the half-reactions one at a time and combine them so that electrons are neither created nor destroyed in the reaction. Instructions To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Enter an equation of a chemical reaction and click Balance.
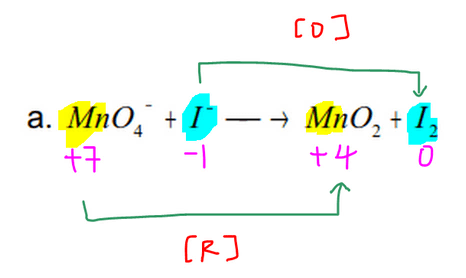
Balance atoms that change oxidation state. An unbalanced redox reaction can be balanced using this calculator. Something is oxidized and something else is reduced.
One for oxidation and one for reduction. Fe Au Co Br C O N F. We know that redox reactions are ones that involve electron transfer.
Determine number of electrons gained or lost. The answer will appear below. To predict the product of a reaction the equation balancer is also available on the internet where all you have to do is to enter the reactants and the complete final equation would be displayed in a few seconds.
Balancing Redox Reactions A reaction in which a reducing agent loses electrons while it is oxidized and the oxidizing agent gains electrons while it is reduced is called as redox oxidation reduction reaction. Use uppercase for the first character in the element and lowercase for the second character. Redox processes in the basic medium involves having hydroxide ions OH- to be balanced as bases liberate into OH- ions.